Structural Properties and Macrophage Activation of Cell Wall Polysaccharides from the Fruiting Bodies of Hericium erinaceus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Fruiting Body Cultivation and Selection
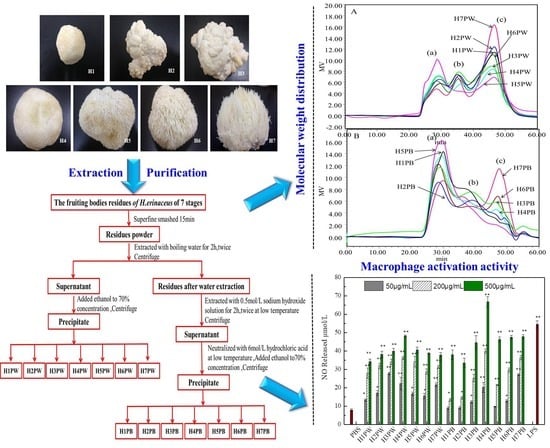
2.3. Extraction of H. erinaceus Cell Wall Polysaccharides at Seven Developmental Stages
2.4. Physicochemical Properties and Structural Characterization of Cell Wall Polysaccharides
2.5. Macrophage Activation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Yields and Contents of H. erinaceus Cell Wall Polysaccharides at Seven Developmental Stages
3.2. Molecular Weight Distribution of Cell Wall Polysaccharides of H. erinaceus at Different Developmental Stages
3.3. Monosaccharide Compositions of Cell Wall Polysaccharides of H. erinaceus at Different Developmental Stages
3.4. Macrophage Activation Activity of Cell Wall Polysaccharides of H. erinaceus at Different Developmental Stages
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.; Tania, M.; Liu, R.; Rahman, M.M. Hericium erinaceus: An edible mushroom with medicinal values. J. Complement. Integr. Med. 2013, 10, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.J.; Li, Y.H.; Zan, X.Y.; Yang, Y.; Sun, W.J.; Qian, J.Y.; Zhou, Q.; Yu, S.L. Purification and partial characterization of a novel hemagglutinating glycoprotein from the cultured mycelia of Hericium erinaceus. Process Biochem. 2014, 49, 1362–1369. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, nutrition, and health-promoting properties of Hericium erinaceus (lion’s mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef] [PubMed]
- Csóka, M.; Geosel, A.; Amtmann, M.; Korany, K. Volatile Composition of Some Cultivated and Wild Culinary-Medicinal Mushrooms from Hungary. Int. J. Med. Mushrooms 2017, 19, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium erinaceus, an amazing medicinal mushroom. Mycol. Prog. 2015, 14, 91. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.; Li, Q.; Zhao, T.; Zhang, M.; Feng, W.; Takase, M.; Wu, X.; Zhou, Z.; Yang, L.; et al. Preparation, characterization, and anti-Helicobacter pylori activity of Bi3+-Hericium erinaceus polysaccharide complex. Carbohydr. Polym. 2014, 110, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lv, G.; Pan, H.; Pandey, A.; He, W.; Fan, L. Antioxidant and hepatoprotective potential of endo-polysaccharides from Hericium erinaceus grown on tofu whey. Int. J. Biol. Macromol. 2012, 51, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Cho, J.Y.; Hong, E.K. Study on macrophage activation and structural characteristics of purified polysaccharides from the liquid culture broth of Hericium erinaceus. Carbohyd. Polym. 2009, 78, 162–168. [Google Scholar] [CrossRef]
- Kim, S.P.; Kang, M.Y.; Kim, J.H.; Nam, S.H.; Friedman, M. Composition and mechanism of antitumor effects of Hericium erinaceus mushroom extracts in tumor-bearing mice. J. Agric. Food Chem. 2011, 59, 9861–9869. [Google Scholar] [CrossRef] [PubMed]
- Zan, X.; Cui, F.; Li, Y.; Yang, Y.; Wu, D.; Sun, W.; Ping, L. Hericium erinaceus polysaccharide-protein HEG-5 inhibits SGC-7901 cell growth via cell cycle arrest and apoptosis. Int. J. Biol. Macromol. 2015, 76, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, Y.; Xu, D.; Konishi, T.; Gao, Q. Hericium erinaceus (Yamabushitake): A unique resource for developing functional foods and medicines. Food Funct. 2014, 5, 3055–3064. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [PubMed]
- Gern, R.M.M.; Wisbeck, E.; Rampinelli, J.R.; Ninow, J.L. Furlan, S.A. Alternative medium for production of Pleurotus ostreatus biomass and potential antitumor polysaccharides. Bioresour. Technol. 2008, 99, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, W.; Lin, S. Cheung, P.C. Cell wall structure of mushroom sclerotium (Pleurotus tuber regium): Part 1. Fractionation and characterization of soluble cell wall polysaccharides. Food Hydrocoll. 2014, 36, 189–195. [Google Scholar] [CrossRef]
- Leung, M.Y.; Liu, C.; Koon, J.C.; Fung, K.P. Polysaccharide biological response modifiers. Immunol. Lett. 2006, 105, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K.; Tanaka, M.; Matsumoto, K.; Yamaguchi, Y.; Shimomura, M.; Miyauchi, S. β-Glucane Extracted from Pycnoporus Coccineus by Hot Compressed Water and Sodium Hydroxide Solution. Kobunshi Ronbunshu 2010, 67, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Park, C.; Abuorf, M.M.; Novak, J.T. Analysis of floc structure and predicting sludge digestibility using different cation-associated eps extraction methods. Proc. Water Environ. Fed. 2004, 8, 21–37. [Google Scholar] [CrossRef]
- Wang, X.; Chi, Y.L.; Hua, C.; Wang, Z.J.; Wu, Y.L.; Jiang, H.T.; Zhou, F.; Wang, R. Effect of different extraction methods on the feature of polysaccharide in Cordyceps Militaris. Sci. Technol. Food Ind. 2015, 36, 49–56. [Google Scholar]
- Dikeman, C.L.; Bauer, L.L.; Flickinger, E.A.; Fahey, G.C. Effects of stage of maturity and cooking on the chemical composition of select mushroom varieties. J. Agric. Food Chem. 2005, 53, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Li, Y.; Yang, Y.; Sun, W.; Wu, D.; Ping, L. Changes in chemical components and cytotoxicity at different maturity stages of Pleurotus eryngii fruiting body. J. Agric. Food Chem. 2014, 62, 12631–12640. [Google Scholar] [CrossRef] [PubMed]
- Oguri, S.; Ando, A.; Nagata, Y. A novel developmental stage-specific lectin of the basidiomycete Pleurotus cornucopiae. J. Bacteriol. 1996, 178, 5692–5698. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Z.; Wu, D.; Chen, X.; Zhou, S.; Liu, Y.; Yang, Y.; Cui, F. Chemical compositions and macrophage activation of polysaccharides from Leon's mane culinary-medicinal mushroom Hericium erinaceus (higher basidiomycetes) in different maturation stages. Int. J. Med. Mushrooms 2015, 17, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Z.; Wu, D.; Zhou, S.; Liu, Y.F.; Li, Z.P.; Feng, J.; Yang, Y. Structure elucidation of a bioactive polysaccharide from fruiting bodies of Hericium erinaceus in different maturation stages. Carbohydr. Polym. 2016, 144, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.N. A beta-D-glucan isolated from the fruiting bodies of Hericium erinaceus and its aqueous conformation. Carbohydr. Res. 2006, 341, 791–795. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Chen, Y.C.; Shen, S.C.; Chen, L.G.; Lee, T.J.; Yang, L.L. Wogonin, baicalin, and baicalein inhibition of inducible nitric oxide synthase and cyclooxygenase-2 gene expressions induced by nitric oxide synthase inhibitors and lipopolysaccharide. Biochem. Pharmacol. 2001, 61, 1417–1427. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Wu, T.P.; Huang, S.J.; Mau, J.L. Nonvolatile taste components of Agaricus bisporus harvested at different stages of maturity. Food Chem. 2007, 103, 1457–1464. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Effect of fruiting body maturity stage on chemical composition and antimicrobial activity of Lactarius sp. mushrooms. J. Agric. Food Chem. 2007, 55, 8766–8771. [Google Scholar] [CrossRef] [PubMed]
- Volman, J.J.; Helsper, J.P.; Wei, S.; Baars, J.J.; van Griensven, L.J.; Sonnenberg, A.S.; Mensink, R.P.; Plat, J. Effects of mushroom-derived β-glucan-rich polysaccharide extracts on nitric oxide production by bone marrow-derived macrophages and nuclear factor-κB transactivation in Caco-2 reporter cells: Can effects be explained by structure? Mol. Nutr. Food Res. 2010, 54, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Mourão, F.; Umeo, S.H.; Takemura, O.S.; Linde, G.A.; Colauto, N.B. Antioxidant activity of Agaricus brasiliensis basidiocarps on different maturation phases. Braz. J. Microbiol. 2011, 42, 197–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourão, F.; Linde, G.A.; Messa, V.; Cunha, P.L., Jr.; Silva, A.V.; Eira, A.F.; Colauto, N.B. Antineoplasic activity of Agaricus brasiliensis basidiocarps on different maturation phases. Braz. J. Microbiol. 2009, 40, 901–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Peak a | Peak b | Peak c | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mw (Da) | Mw/Mn | Ratio (%) | Mw (Da) | Mw/Mn | Ratio (%) | Mw (Da) | Mw/Mn | Ratio (%) | |
| H1PW | 1.19 × 107 | 1.21 | 23.2 | 2.09 × 106 | 1.20 | 25.1 | 3.17 × 105 | 1.49 | 51.7 |
| H2PW | 8.77 × 106 | 1.24 | 27.9 | 1.73 × 106 | 1.10 | 24.1 | 3.18 × 105 | 1.44 | 48.0 |
| H3PW | 1.07 × 107 | 1.30 | 29.2 | 2.26 × 106 | 1.22 | 30.4 | 4.12 × 105 | 1.25 | 40.4 |
| H4PW | 1.08 × 107 | 1.34 | 38.0 | 2.58 × 106 | 1.11 | 22.8 | 6.14 × 105 | 1.27 | 39.1 |
| H5PW | 1.28 × 107 | 1.13 | 47.7 | 3.54 × 106 | 1.08 | 17.9 | 8.13 × 105 | 1.30 | 34.3 |
| H6PW | 1.45 × 107 | 1.22 | 28.0 | 2.70 × 106 | 1.12 | 23.3 | 5.11 × 105 | 1.41 | 48.6 |
| H7PW | 1.98 × 107 | 1.24 | 19.2 | 3.85 × 106 | 1.16 | 23.4 | 4.53 × 105 | 1.80 | 57.4 |
| H1PB | 2.10 × 106 | 1.29 | 53.5 | 4.00 × 105 | 1.28 | 39.0 | 1.02 × 105 | 1.02 | 7.5 |
| H2PB | 2.74 × 106 | 1.40 | 46.4 | 4.18 × 105 | 1.31 | 42.0 | 1.15 × 105 | 1.02 | 11.6 |
| H3PB | 2.41 × 106 | 1.38 | 51.3 | 4.02 × 105 | 1.19 | 37.3 | 1.42 × 105 | 1.02 | 11.4 |
| H4PB | 2.46 × 106 | 1.27 | 60.7 | 4.72 × 105 | 1.22 | 28.4 | 1.05 × 105 | 1.08 | 10.9 |
| H5PB | 2.11 × 106 | 1.34 | 71.1 | 5.11 × 105 | 1.02 | 24.2 | 3.52 × 105 | 1.01 | 4.6 |
| H6PB | 2.37 × 106 | 1.29 | 55.2 | 4.57 × 105 | 1.13 | 27.7 | 1.37 × 105 | 1.09 | 17.2 |
| H7PB | 1.76 × 106 | 1.31 | 40.5 | 3.43 × 105 | 1.18 | 29.8 | 4.79 × 104 | 1.44 | 29.7 |
| Sample | Fucose | Galactose | Glucose | Glucuronic Acid |
|---|---|---|---|---|
| H1PW | --- | 334.27 | 2182.25 | 92.96 |
| H2PW | 90.72 | 554.20 | 1886.96 | 78.09 |
| H3PW | --- | 276.65 | 2093.30 | 80.32 |
| H4PW | --- | 265.97 | 2195.48 | 78.25 |
| H5PW | --- | 153.70 | 2126.49 | 43.85 |
| H6PW | 94.53 | 355.30 | 1917.93 | 109.10 |
| H7PW | 106.80 | 491.47 | 1914.19 | 102.82 |
| H1PB | --- | --- | 2080.93 | --- |
| H2PB | --- | --- | 1625.06 | --- |
| H3PB | --- | --- | 2030.18 | --- |
| H4PB | --- | --- | 1826.03 | --- |
| H5PB | --- | --- | 1937.56 | --- |
| H6PB | --- | --- | 1778.14 | --- |
| H7PB | --- | --- | 1996.60 | --- |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Yang, S.; Tang, C.; Liu, Y.; Li, Q.; Zhang, H.; Cui, F.; Yang, Y. Structural Properties and Macrophage Activation of Cell Wall Polysaccharides from the Fruiting Bodies of Hericium erinaceus. Polymers 2018, 10, 850. https://doi.org/10.3390/polym10080850
Wu D, Yang S, Tang C, Liu Y, Li Q, Zhang H, Cui F, Yang Y. Structural Properties and Macrophage Activation of Cell Wall Polysaccharides from the Fruiting Bodies of Hericium erinaceus. Polymers. 2018; 10(8):850. https://doi.org/10.3390/polym10080850
Chicago/Turabian StyleWu, Di, Shan Yang, Chuan Tang, Yanfang Liu, Qiaozhen Li, Henan Zhang, Fengjie Cui, and Yan Yang. 2018. "Structural Properties and Macrophage Activation of Cell Wall Polysaccharides from the Fruiting Bodies of Hericium erinaceus" Polymers 10, no. 8: 850. https://doi.org/10.3390/polym10080850
APA StyleWu, D., Yang, S., Tang, C., Liu, Y., Li, Q., Zhang, H., Cui, F., & Yang, Y. (2018). Structural Properties and Macrophage Activation of Cell Wall Polysaccharides from the Fruiting Bodies of Hericium erinaceus. Polymers, 10(8), 850. https://doi.org/10.3390/polym10080850