Recent Advances in Organic Thermoelectric Materials: Principle Mechanisms and Emerging Carbon-Based Green Energy Materials
Abstract
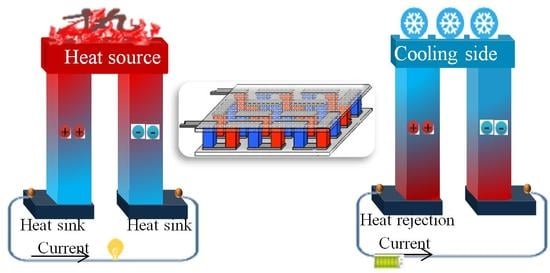
:1. Introduction
2. Principle Mechanisms of Thermoelectric Materials
2.1. Electrical Conductivity
2.2. Seebeck Coefficient
2.3. Thermal Conductivity
3. Carbon-Based Organic Thermoelectric Materials
3.1. CNT-Based Organic Thermoelectric Materials
3.1.1. p-Type CNT-Based Organic Thermoelectric Materials
3.1.2. n-Type CNT-Based Organic Thermoelectric Materials
3.2. Graphite and Its Derivate-Based Organic Thermoelectric Materials
3.2.1. Graphene-Based Organic Thermoelectric Materials
3.2.2. Graphite- and Expanded-Graphite-Based Organic Thermoelectric Materials
3.3. Ternary Thermoelectric Composites
4. Conclusions and Prospects
Funding
Conflicts of Interest
References
- Date, A.; Date, A.; Dixon, C.; Akbarzadeh, A. Progress of thermoelectric power generation systems: Prospect for small to medium scale power generation. Renew. Sustain. Energy Rev. 2014, 33, 371–381. [Google Scholar] [CrossRef]
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.; Minnich, A.; Yu, B.; Yan, X.; Wang, D.; Muto, A.; Vashaee, D.; et al. High-Thermoelectric Performance of Nanostructured Bismuth Antimony Telluride Bulk Allys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.Y.; Hogan, T.P.; Rocci-Lane, M.; Brazis, P.; Ireland, J.R.; Kannewurf, C.R.; Bastea, M.; Uher, C.; Kanatzidis, M.G. A new thermoelectric material: CsBi4Te6. J. Am. Chem. Soc. 2004, 126, 6414–6428. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chau, K.T. Thermoelectric automotive waste heat energy recovery using maximum power point tracking. Energy Convers. Manag. 2009, 50, 1506–1512. [Google Scholar] [CrossRef]
- Hatami, M.; Ganji, D.D.; Gorji-Bandpy, M. A review of different heat exchangers designs for increasing the diesel exhaust waste heat recovery. Renew. Sustain. Energy Rev. 2014, 37, 168–181. [Google Scholar] [CrossRef]
- Saidur, R.; Rezaei, M.; Muzammil, W.K.; Hassan, M.H.; Paria, S.; Hasanuzzaman, M. Technologies to recover exhaust heat from internal combustion engines. Renew. Sustain. Energy Rev. 2012, 16, 5649–5659. [Google Scholar] [CrossRef]
- Culebras, M.; Choi, K.; Cho, C. Recent Progress in Flexible Organic Thermoelectrics. Micromachines 2018, 9, 638. [Google Scholar] [CrossRef]
- Bell, L.E. Cooling, heating, generating power, and recovering waste heat with thermoelectric systems. Science 2008, 321, 1457–1461. [Google Scholar] [CrossRef]
- Champier, D. Thermoelectric generators: A review of applications. Energy Convers. Manag. 2017, 140, 167–181. [Google Scholar] [CrossRef]
- Zheng, X.F.; Yan, Y.Y.; Simpson, K. A potential candidate for the sustainable and reliable domestic energy generation-Thermoelectric cogeneration system. Appl. Therm. Eng. 2013, 53, 305–311. [Google Scholar] [CrossRef]
- Hasan Nia, M.; Abbas Nejad, A.; Goudarzi, A.M.; Valizadeh, M.; Samadian, P. Cogeneration solar system using thermoelectric module and fresnel lens. Energy Convers. Manag. 2014, 84, 305–310. [Google Scholar] [CrossRef]
- Kim, S.J.; We, J.H.; Cho, B.J. A wearable thermoelectric generator fabricated on a glass fabric. Energy Environ. Sci. 2014, 7, 1959–1965. [Google Scholar] [CrossRef]
- Du, F.P.; Qiao, X.; Wu, Y.G.; Fu, P.; Liu, S.P.; Zhang, Y.F.; Wang, Q.Y. Fabrication of porous polyvinylidene fluoride/multi-walled carbon nanotube nanocomposites and their enhanced thermoelectric performance. Polymers 2018, 10, 797. [Google Scholar] [CrossRef]
- Zhang, Q.; Chere, E.K.; Sun, J.; Cao, F.; Dahal, K.; Chen, S.; Chen, G.; Ren, Z. Studies on Thermoelectric Properties of n-type Polycrystalline SnSe1−xSx by Iodine Doping. Adv. Energy Mater. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Zhao, L.-D.; Lo, S.-H.; Zhang, Y.; Sun, H.; Tan, G.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 2014, 508, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, B.; Heremans, J.P.; Zhao, J.C. High-temperature oxidation behavior of thermoelectric SnSe. J. Alloys Compd. 2016, 669, 224–231. [Google Scholar] [CrossRef] [Green Version]
- Scheele, M.; Oeschler, N.; Meier, K.; Kornowski, A.; Klinke, C.; Weller, H. Synthesis and thermoelectric characterization of Bi2Te3 nanoparticles. Adv. Funct. Mater. 2009, 19, 3476–3483. [Google Scholar] [CrossRef]
- Yu, C.X.; Zhang, G.; Zhang, Y.W.; Peng, L.M. Strain engineering on the thermal conductivity and heat flux of thermoelectric Bi2Te3 nanofilm. Nano Energy 2015, 17, 104–110. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Moon, W.; Berger, A.; Lee, J. Enhanced seebeck coefficients of thermoelectric Bi2Te3 nanowires as a result of an optimized annealing process. J. Phys. Chem. C 2012, 116, 19512–19516. [Google Scholar] [CrossRef]
- Sun, Z.; Liufu, S.; Chen, X.; Chen, L. Tellurization: An alternative strategy to construct thermoelectric Bi2Te3 films. J. Phys. Chem. C 2011, 115, 16167–16171. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, J.; Katz, H.E.; Fang, F.; Opila, R.L. Promising thermoelectric properties of commercial PEDOT:PSS materials and their Bi2Te3 powder composites. ACS Appl. Mater. Interfaces 2010, 2, 3170–3178. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.S.; Jesser, W.A.; Rosi, F.D. Microstructure of thermoelectric SiGe alloys containing fullerite. Mater. Sci. Eng. B 1995, 33, 195–203. [Google Scholar] [CrossRef]
- Lee, E.K.; Yin, L.; Lee, Y.; Lee, J.W.; Lee, S.J.; Lee, J.; Cha, S.N.; Whang, D.; Hwang, G.S.; Hippalgaonkar, K.; et al. Large thermoelectric figure-of-merits from SiGe nanowires by simultaneously measuring electrical and thermal transport properties. Nano Lett. 2012, 12, 2918–2923. [Google Scholar] [CrossRef] [PubMed]
- Strasser, M.; Aigner, R.; Franosch, M.; Wachutka, G. Miniaturized thermoelectric generators based on poly-Si and poly-SiGe surface micromachining. Sens. Actuators A Phys. 2002, 97–98, 535–542. [Google Scholar] [CrossRef]
- Guo, R.; Wang, X.; Kuang, Y.; Huang, B. First-principles study of anisotropic thermoelectric transport properties of IV-VI semiconductor compounds SnSe and SnS. Phys. Rev. B Condens. Matter Mater. Phys. 2015, 92, 115202. [Google Scholar] [CrossRef]
- Han, Y.M.; Zhao, J.; Zhou, M.; Jiang, X.X.; Leng, H.Q.; Li, L.F. Thermoelectric performance of SnS and SnS-SnSe solid solution. J. Mater. Chem. A 2015, 3, 4555–4559. [Google Scholar] [CrossRef]
- Tan, Q.; Zhao, L.D.; Li, J.F.; Wu, C.F.; Wei, T.R.; Xing, Z.B.; Kanatzidis, M.G. Thermoelectrics with earth abundant elements: Low thermal conductivity and high thermopower in doped SnS. J. Mater. Chem. A 2014, 2, 17302–17306. [Google Scholar] [CrossRef]
- Toprak, M.S.; Stiewe, C.; Platzek, D.; Williams, S.; Bertini, L.; Müller, E.; Gatti, C.; Zhang, Y.; Rowe, M.; Muhammed, M. The impact of nanostructuring on the thermal conductivity of thermoelectric CoSb3. Adv. Funct. Mater. 2004, 14, 1189–1196. [Google Scholar] [CrossRef]
- Du, Y.; Niu, H.; Li, J.; Dou, Y.; Shen, S.; Jia, R.; Xu, J. Morphologies Tuning of Polypyrrole and Thermoelectric Properties of Polypyrrole Nanowire/Graphene Composites. Polymers 2018, 10, 1143. [Google Scholar] [CrossRef]
- Wan, C.; Gu, X.; Dang, F.; Itoh, T.; Wang, Y.; Sasaki, H.; Kondo, M.; Koga, K.; Yabuki, K.; Snyder, G.J.; et al. Flexible n-type thermoelectric materials by organic intercalation of layered transition metal dichalcogenide TiS2. Nat. Mater. 2015, 14, 622–627. [Google Scholar] [CrossRef]
- Toshima, N.; Jiravanichanun, N.; Marutani, H. Organic thermoelectric materials composed of conducting polymers and metal nanoparticles. J. Electron. Mater. 2012, 41, 1735–1742. [Google Scholar] [CrossRef]
- Nonoguchi, Y.; Sato, D.; Kawai, T. Crystallinity-dependent thermoelectric properties of a two-dimensional coordination polymer: Ni3(2,3,6,7,10,11-hexaiminotriphenylene)2. Polymers 2018, 10, 962. [Google Scholar] [CrossRef]
- Shi, W.; Chen, J.; Xi, J.; Wang, D.; Shuai, Z. Search for organic thermoelectric materials with high mobility: The case of 2,7-dialkyl[1]benzothieno[3,2-b][1]benzothiophene derivatives. Chem. Mater. 2014, 26, 2669–2677. [Google Scholar] [CrossRef]
- Jang, W.; Cho, H.; Choi, K.; Park, Y. Manipulation of p-/n-Type Thermoelectric Thin Films through a Layer-by-Layer Assembled Carbonaceous Multilayer Structure. Micromachines 2018, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Luceño Sánchez, J.; Peña Capilla, R.; Díez-Pascual, A. High-Performance PEDOT:PSS/Hexamethylene Diisocyanate-Functionalized Graphene Oxide Nanocomposites: Preparation and Properties. Polymers 2018, 10, 1169. [Google Scholar] [CrossRef]
- Weathers, A.; Khan, Z.U.; Brooke, R.; Evans, D.; Pettes, M.T.; Andreasen, J.W.; Crispin, X.; Shi, L. Significant electronic thermal transport in the conducting polymer poly(3,4-ethylenedioxythiophene). Adv. Mater. 2015, 27, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Petsagkourakis, I.; Pavlopoulou, E.; Portale, G.; Kuropatwa, B.A.; Dilhaire, S.; Fleury, G.; Hadziioannou, G. Structurally-driven enhancement of thermoelectric properties within poly(3,4-ethylenedioxythiophene) thin films. Sci. Rep. 2016, 6, 30501. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, M.; Seong, H.G.; Jung, J.Y.; Baeck, S.H.; Shim, S.E. Roles of silica-coated layer on graphite for thermal conductivity, heat dissipation, thermal stability, and electrical resistivity of polymer composites. Polymer 2018, 148, 295–302. [Google Scholar] [CrossRef]
- Khan, Z.U.; Bubnova, O.; Jafari, M.J.; Brooke, R.; Liu, X.; Gabrielsson, R.; Ederth, T.; Evans, D.R.; Andreasen, J.W.; Fahlman, M.; et al. Acido-basic control of the thermoelectric properties of poly(3,4-ethylenedioxythiophene)tosylate (PEDOT-Tos) thin films. J. Mater. Chem. C 2015, 3, 10616–10623. [Google Scholar] [CrossRef] [Green Version]
- Kroon, R.; Mengistie, D.A.; Kiefer, D.; Hynynen, J.; Ryan, J.D.; Yu, L.; Müller, C. Thermoelectric plastics: From design to synthesis, processing and structure-property relationships. Chem. Soc. Rev. 2016, 45, 6147–6164. [Google Scholar] [CrossRef]
- Wang, H.L.; Wang, M.X.; Qian, C.; Hong, X.K.; Zhang, D.B.; Liu, Y.S.; Yang, X.F. Spin thermoelectric effects in organic single-molecule devices. Phys. Lett. A 2017, 381, 1738–1744. [Google Scholar] [CrossRef]
- Roussel, F.; Chen Yu King, R.; Kuriakose, M.; Depriester, M.; Hadj-Sahraoui, A.; Gors, C.; Addad, A.; Brun, J.F. Electrical and thermal transport properties of polyaniline/silver composites and their use as thermoelectric materials. Synth. Met. 2015, 199, 196–204. [Google Scholar] [CrossRef]
- Horta-Romarís, L.; González-Rodríguez, M.V.; Lasagabáster, A.; Rivadulla, F.; Abad, M.J. Thermoelectric properties and intrinsic conduction processes in DBSA and NaSIPA doped polyanilines. Synth. Met. 2018, 243, 44–50. [Google Scholar] [CrossRef]
- Glaudell, A.M.; Cochran, J.E.; Patel, S.N.; Chabinyc, M.L. Impact of the doping method on conductivity and thermopower in semiconducting polythiophenes. Adv. Energy Mater. 2015, 5, 1401072. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Zhang, Z.; Wang, B.; Qiu, J.; Hui, D.; Wang, S. Polymer composites-based thermoelectric materials and devices. Compos. Part B Eng. 2017, 122, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Ma, H.; Tian, Z. Thermoelectric properties of crystalline and amorphous polypyrrole: A computational study. Appl. Therm. Eng. 2017, 111, 1441–1447. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Y.; Pei, W.B.; Huang, L.; Xu, W.; Zhang, Q. Polypyrrole nanotube film for flexible thermoelectric application. Synth. Met. 2014, 196, 173–177. [Google Scholar] [CrossRef]
- Oshima, K.; Sadakata, S.; Asano, H.; Shiraishi, Y.; Toshima, N. Thermostability of hybrid thermoelectric materials consisting of poly(Ni-ethenetetrathiolate), polyimide and carbon nanotubes. Materials 2017, 10, 824. [Google Scholar] [CrossRef]
- Li, J.; Lai, C.; Jia, X.; Wang, L.; Xiang, X.; Ho, C.L.; Li, H.; Wong, W.Y. Effect of electron donor/acceptor substituents on the Seebeck coefficient and thermoelectric properties of poly(3-methylthiophene methine)s/graphite composites. Compos. Part B Eng. 2015, 77, 248–256. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, D.; Zhu, Z.; Liu, E.; Lu, B.; Xu, J.; Zhao, F.; Hou, J.; Liu, H.; Jiang, F. Electrochemical Treatment for Effectively Tuning Thermoelectric Properties of Free-Standing Poly(3-methylthiophene) Films. ChemPhysChem 2016, 2256–2262. [Google Scholar] [CrossRef]
- Kymakis, E.; Amaratunga, G.A.J. Electrical properties of single-wall carbon nanotube-polymer composite films. J. Appl. Phys. 2006, 99, 084302. [Google Scholar] [CrossRef]
- Hong, C.T.; Lee, W.; Kang, Y.H.; Yoo, Y.; Ryu, J.; Cho, S.Y.; Jang, K.S. Effective doping by spin-coating and enhanced thermoelectric power factors in SWCNT/P3HT hybrid films. J. Mater. Chem. A 2015, 3, 12314–12319. [Google Scholar] [CrossRef]
- Dun, C.; Hewitt, C.A.; Huang, H.; Xu, J.; Zhou, C.; Huang, W.; Cui, Y.; Zhou, W.; Jiang, Q.; Carroll, D.L. Flexible n-type thermoelectric films based on Cu-doped Bi2Se3 nanoplate and Polyvinylidene Fluoride composite with decoupled Seebeck coefficient and electrical conductivity. Nano Energy 2015, 18, 306–314. [Google Scholar] [CrossRef]
- Dun, C.; Hewitt, C.A.; Huang, H.; Xu, J.; Montgomery, D.S.; Nie, W.; Jiang, Q.; Carroll, D.L. Layered Bi2Se3 nanoplate/polyvinylidene fluoride composite based n-type thermoelectric fabrics. ACS Appl. Mater. Interfaces 2015, 7, 7054–7059. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Dun, C.; Ge, B.; Wang, K.; Shi, Z.; Liu, G.; Carroll, D.L.; Qiao, G. Highly robust and flexible n-type thermoelectric film based on Ag2Te nanoshuttle/polyvinylidene fluoride hybrids. Nanoscale 2018, 10, 14830–14834. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kang, Y.H.; Lee, J.Y.; Jang, K.S.; Cho, S.Y. Hot-pressing for improving performance of CNT/conjugated polymer thermoelectric films and power generators. Mater. Today Commun. 2017, 10, 41–45. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Mo, J.-H.; Kang, Y.H.; Cho, S.Y.; Jang, K.-S. Thermoelectric fibers from well-dispersed carbon nanotube/poly(vinyliedene fluoride) pastes for fiber-based thermoelectric generators. Nanoscale 2018, 10, 19766–19773. [Google Scholar] [CrossRef]
- Du, Y.; Shen, S.Z.; Cai, K.; Casey, P.S. Research progress on polymer-inorganic thermoelectric nanocomposite materials. Prog. Polym. Sci. 2012, 37, 820–841. [Google Scholar] [CrossRef]
- Lévesque, I.; Gao, X.; Klug, D.D.; Tse, J.S.; Ratcliffe, C.I.; Leclerc, M. Highly soluble poly(2,7-carbazolenevinylene) for thermoelectrical applications: From theory to experiment. React. Funct. Polym. 2005, 65, 23–36. [Google Scholar] [CrossRef]
- Lévesque, I.; Bertrand, P.O.; Blouin, N.; Leclerc, M.; Zecchin, S.; Zotti, G.; Ratcliffe, C.I.; Klug, D.D.; Gao, X.; Gao, F.; et al. Synthesis and thermoelectric properties of polycarbazole, polyindolocarbazole, and polydiindolocarbazole derivatives. Chem. Mater. 2007, 19, 2128–2138. [Google Scholar] [CrossRef]
- Yu, C.; Choi, K.; Yin, L.; Grunlan, J.C. Light-weight flexible carbon nanotube based organic composites with large thermoelectric power factors. ACS Nano 2011, 5, 7885–7892. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yi, S.I.; Pu, X.; Yu, C. Simultaneously improving electrical conductivity and thermopower of polyaniline composites by utilizing carbon nanotubes as high mobility conduits. ACS Appl. Mater. Interfaces 2015, 7, 9589–9597. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gittleson, F.; Carmo, M.; Sekol, R.C.; Taylor, A.D. Scalable fabrication of multifunctional freestanding carbon nanotube/polymer composite thin films for energy conversion. ACS Nano 2012, 6, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Ashori, A.; Menbari, S.; Bahrami, R. Mechanical and thermo-mechanical properties of short carbon fiber reinforced polypropylene composites using exfoliated graphene nanoplatelets coating. J. Ind. Eng. Chem. 2016, 38, 37–42. [Google Scholar] [CrossRef]
- Lu, X.; Morelli, D.T.; Xia, Y.; Ozolins, V. Increasing the thermoelectric figure of merit of tetrahedrites by Co-doping with nickel and zinc. Chem. Mater. 2015, 27, 408–413. [Google Scholar] [CrossRef]
- Olvera, A.A.; Moroz, N.A.; Sahoo, P.; Ren, P.; Bailey, T.P.; Page, A.A.; Uher, C.; Poudeu, P.F.P. Partial indium solubility induces chemical stability and colossal thermoelectric figure of merit in Cu2Se. Energy Environ. Sci. 2017, 10, 1668–1676. [Google Scholar] [CrossRef]
- Fahrnbauer, F.; Souchay, D.; Wagner, G.; Oeckler, O. High Thermoelectric Figure of Merit Values of Germanium Antimony Tellurides with Kinetically Stable Cobalt Germanide Precipitates. J. Am. Chem. Soc. 2015, 137, 12633–12638. [Google Scholar] [CrossRef]
- Perumal, S.; Roychowdhury, S.; Biswas, K. Reduction of thermal conductivity through nanostructuring enhances the thermoelectric figure of merit in Ge1-xBixTe. Inorg. Chem. Front. 2016, 3, 125–132. [Google Scholar] [CrossRef]
- Qu, S.; Ming, C.; Yao, Q.; Lu, W.; Zeng, K.; Shi, W.; Shi, X.; Uher, C.; Chen, L. Understanding the Intrinsic Carrier Transport in Highly Oriented Poly(3-hexylthiophene): Effect of Side Chain Regioregularity. Polymers 2018, 10, 815. [Google Scholar] [CrossRef]
- Cho, C.; Stevens, B.; Hsu, J.H.; Bureau, R.; Hagen, D.A.; Regev, O.; Yu, C.; Grunlan, J.C. Completely organic multilayer thin film with thermoelectric power factor rivaling inorganic tellurides. Adv. Mater. 2015, 27, 2996–3001. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Xu, W.; Zhu, D. Organic Thermoelectric Materials: Emerging Green Energy Materials Converting Heat to Electricity Directly and Efficiently. Adv. Mater. 2014, 26, 6829–6851. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Fan, Z.; Cheng, H.; Guan, X.; Wang, C.; Sun, K.; Ouyang, J. Recent Development of Thermoelectric Polymers and Composites. Macromol. Rapid Commun. 2018, 39, 1–22. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Neto, P.H.; Da Silva Filho, D.A.; Roncaratti, L.F.; Acioli, P.H.; e Silva, G.M. Low-Temperature Seebeck Coefficients for Polaron-Driven Thermoelectric Effect in Organic Polymers. J. Phys. Chem. A 2016, 120, 4923–4927. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Lu, K.F.; Tseng, C.Y. Carrier drift velocity balance mechanism in Si-based thin film solar cells using graded microcrystalline SiGe absorption layer. Sol. Energy 2015, 114, 1–7. [Google Scholar] [CrossRef]
- Mao, J.; Shuai, J.; Song, S.; Wu, Y.; Dally, R.; Zhou, J.; Liu, Z.; Sun, J.; Zhang, Q.; dela Cruz, C.; et al. Manipulation of ionized impurity scattering for achieving high thermoelectric performance in n-type Mg3Sb2-based materials. Proc. Natl. Acad. Sci. USA 2017, 114, 10548–10553. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Du, D.; Guan, X.; Ouyang, J. Polymer films with ultrahigh thermoelectric properties arising from significant seebeck coefficient enhancement by ion accumulation on surface. Nano Energy 2018, 51, 481–488. [Google Scholar] [CrossRef]
- Wang, H.; Ail, U.; Gabrielsson, R.; Berggren, M.; Crispin, X. Ionic Seebeck effect in conducting polymers. Adv. Energy Mater. 2015, 5, 1500044. [Google Scholar] [CrossRef]
- Broch, K.; Venkateshvaran, D.; Lemaur, V.; Olivier, Y.; Beljonne, D.; Zelazny, M.; Nasrallah, I.; Harkin, D.J.; Statz, M.; Di Pietro, R.; et al. Measurements of Ambipolar Seebeck Coefficients in High-Mobility Diketopyrrolopyrrole Donor–Acceptor Copolymers. Adv. Electron. Mater. 2017, 3, 225. [Google Scholar] [CrossRef]
- Zhang, Y.; Choi, J.R.; Park, S.J. Interlayer polymerization in amine-terminated macromolecular chain-grafted expanded graphite for fabricating highly thermal conductive and physically strong thermoset composites for thermal management applications. Compos. Part A Appl. Sci. Manuf. 2018, 109, 498–506. [Google Scholar] [CrossRef]
- Zhang, Y.; Heo, Y.-J.; Son, Y.-R.; In, I.; An, K.-H.; Kim, B.-J.; Park, S.-J. Recent advanced thermal interfacial materials: A review of conducting mechanisms and parameters of carbon materials. Carbon 2019, 142, 445–460. [Google Scholar] [CrossRef]
- Zhang, Y.; Choi, J.R.; Park, S.-J. Thermal conductivity and thermo-physical properties of nanodiamond-attached exfoliated hexagonal boron nitride/epoxy nanocomposites for microelectronics. Compos. Part A Appl. Sci. Manuf. 2017, 101, 227–236. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, S.-J. In situ shear-induced mercapto group-activated graphite nanoplatelets for fabricating mechanically strong and thermally conductive elastomer composites for thermal management applications. Compos. Part A Appl. Sci. Manuf. 2018, 112, 40–48. [Google Scholar] [CrossRef]
- Das, A.; Stöckelhuber, K.W.; Jurk, R.; Fritzsche, J.; Klüppel, M.; Heinrich, G. Coupling activity of ionic liquids between diene elastomers and multi-walled carbon nanotubes. Carbon 2009, 47, 3313–3321. [Google Scholar] [CrossRef]
- Kunova, O.; Kustova, E.; Mekhonoshina, M.; Nagnibeda, E. Non-equilibrium kinetics, diffusion and heat transfer in shock heated flows of N2/N and O2/O mixtures. Chem. Phys. 2015, 463, 70–81. [Google Scholar] [CrossRef]
- Hone, J.; Whitney, M.; Piskoti, C.; Zettl, A. Thermal conductivity of single-walled carbon nanotubes. Phys. Rev. B 1999, 59, 2514–2516. [Google Scholar] [CrossRef]
- Burger, N.; Laachachi, A.; Ferriol, M.; Lutz, M.; Toniazzo, V.; Ruch, D. Review of thermal conductivity in composites: Mechanisms, parameters and theory. Prog. Polym. Sci. 2016, 61, 1–28. [Google Scholar] [CrossRef]
- Ye, Z.; Cao, B.; Guo, Z. High and anisotropic thermal conductivity of body-centered tetragonal C4calculated using molecular dynamics. Carbon 2014, 66, 567–575. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Deng, H.; Jing, Y.; Fu, Q. Enhanced thermal conductivity and electrical insulation properties of polymer composites via constructing Pglass/CNTs confined hybrid fillers. Compos. Part A Appl. Sci. Manuf. 2018, 115, 1–7. [Google Scholar] [CrossRef]
- Oh, H.; Kim, K.; Ryu, S.; Kim, J. Enhancement of thermal conductivity of polymethyl methacrylate-coated graphene/epoxy composites using admicellar polymerization with different ionic surfactants. Compos. Part A Appl. Sci. Manuf. 2019, 116, 206–215. [Google Scholar] [CrossRef]
- Ho, C.J.; Chen, M.W.; Li, Z.W. Numerical simulation of natural convection of nanofluid in a square enclosure: Effects due to uncertainties of viscosity and thermal conductivity. Int. J. Heat Mass Transf. 2008, 51, 4506–4516. [Google Scholar] [CrossRef]
- Renteria, J.D.; Ramirez, S.; Malekpour, H.; Alonso, B.; Centeno, A.; Zurutuza, A.; Cocemasov, A.I.; Nika, D.L.; Balandin, A.A. Strongly Anisotropic Thermal Conductivity of Free-Standing Reduced Graphene Oxide Films Annealed at High Temperature. Adv. Funct. Mater. 2015, 25, 4664–4672. [Google Scholar] [CrossRef]
- Saha, B.; Koh, Y.R.; Comparan, J.; Sadasivam, S.; Schroeder, J.L.; Garbrecht, M.; Mohammed, A.; Birch, J.; Fisher, T.; Shakouri, A.; et al. Cross-plane thermal conductivity of (Ti,W)N/(Al,Sc)N metal/semiconductor superlattices. Phys. Rev. B 2016, 93, 045311. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Park, S. Effect of Mercapto-Terminated Silane Treatment on Rheological and Mechanical Properties of Rice Bran Carbon-Reinforced Nitrile Butadiene Rubber Composites. Polym. Compos. 2018, 26, 446–453. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Q.; Li, X.; Lee, D.; Cho, U.R. Comparative study on carboxylated styrene butadiene rubber composites reinforced by hybrid fillers of rice bran carbon and graphite carbon. Carbon Lett. 2018, 27, 72–80. [Google Scholar]
- Zhang, Y.; Rhee, K.Y.; Park, S.-J. Nanodiamond nanocluster-decorated graphene oxide/epoxy nanocomposites with enhanced mechanical behavior and thermal stability. Compos. Part B Eng. 2017, 114, 111–120. [Google Scholar] [CrossRef]
- Fan, Y.; Li, X.; Jang, S.H.; Lee, D.H.; Li, Q.; Cho, U.R. Reinforcement of solution styrene-butadiene rubber by incorporating hybrids of rice bran carbon and surface modified fumed silica. J. Vinyl Addit. Technol. 2018, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, X.; Deng, F.; Li, M.C.; Cho, U.R. Fabrication and characterization of rice bran carbon/styrene butadiene rubber composites fabricated by latex compounding method. Polym. Compos. 2017, 38, 2594–2602. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, F.; Qiu, F.; Lu, C.; Kang, J.; Zhao, D.; Han, S.; Zhuang, X. Cobalt-Doped Porous Carbon Nanosheets Derived from 2D Hypercrosslinked Polymer with CoN4 for High Performance Electrochemical Capacitors. Polymers 2018, 10, 1339. [Google Scholar] [CrossRef]
- Liu, Y.; Park, M.; Shin, H.K.; Pant, B.; Choi, J.; Park, Y.W.; Lee, J.Y.; Park, S.J.; Kim, H.Y. Facile preparation and characterization of poly(vinyl alcohol)/chitosan/graphene oxide biocomposite nanofibers. J. Ind. Eng. Chem. 2014, 20, 4415–4420. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, M.; Zhang, Y.; Liu, M.; Xiong, W.; Liu, S. Large surface area porous carbon materials synthesized by direct carbonization of banana peel and citrate salts for use as high-performance supercapacitors. J. Mater. Sci. Mater. Electron. 2018, 29, 4294–4300. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Ge, X.; Deng, F.; Cho, U.R. Effect of coupling agents and ionic liquid on the properties of rice bran carbon/carboxylated styrene butadiene rubber composites. Macromol. Res. 2015, 23, 952–959. [Google Scholar] [CrossRef]
- Zhang, Y.; Cho, U.R. Enhanced Thermo-Physical Properties of Nitrile-Butadiene Rubber Nanocomposites Filled with Simultaneously Reduced and Functionalized Graphene Oxide. Polym. Compos. 2017, 39, 3227–3235. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, X.; Zeng, Y.; Wang, H.; Lu, Y.; Zhang, J.; Yin, Z.; Chen, Z.; Yang, Y.; Li, L. An Electrochemical Sensor for Diphenylamine Detection Based on Reduced Graphene Oxide/Fe3O4-Molecularly Imprinted Polymer with 1,4-Butanediyl-3,3′-bis-l-vinylimidazolium Dihexafluorophosphate Ionic Liquid as Cross-Linker. Polymers 2018, 10, 1329. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Z.; Xiong, W.; Zhang, Q. Controlled Synthesis of Carbon Nanospheres via the Modulation of the Hydrophilic Length of the Assembled Surfactant Micelles. Langmuir 2018, 34, 10389–10396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Park, S.-J. Enhanced interfacial interaction by grafting carboxylated-macromolecular chains on nanodiamond surfaces for epoxy-based thermosets. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 1890–1898. [Google Scholar] [CrossRef]
- Zhang, Y.; Fei, D.; Xin, G.; Cho, U.-R. Surface modification of novel rice bran carbon functionalized with (3-Mercaptopropyl) trimethoxysilane and its influence on the properties of styrene-butadiene rubber composites. J. Compos. Mater. 2016, 50, 2987–2999. [Google Scholar] [CrossRef]
- Heo, Y.-J.; Lee, J.W.; Son, Y.-R.; Lee, J.-H.; Yeo, C.S.; Lam, T.D.; Park, S.Y.; Park, S.-J.; Sinh, L.H.; Shin, M.K. Large-Scale Conductive Yarns Based on Twistable Korean Traditional Paper (Hanji) for Supercapacitor Applications: Toward High-Performance Paper Supercapacitors. Adv. Energy Mater. 2018, 8, 1801854. [Google Scholar] [CrossRef]
- Zhang, Y.; Rhee, K.Y.; Hui, D.; Park, S.J. A critical review of nanodiamond based nanocomposites: Synthesis, properties and applications. Compos. Part B Eng. 2018, 143, 19–27. [Google Scholar] [CrossRef]
- Kim, K.-W.; Kim, D.-K.; Kim, B.-S.; An, K.-H.; Park, S.-J.; Rhee, K.Y.; Kim, B.-J. Cure behaviors and mechanical properties of carbon fiber-reinforced nylon6/epoxy blended matrix composites. Compos. Part B Eng. 2017, 112, 15–21. [Google Scholar] [CrossRef]
- Mittal, G.; Rhee, K.Y.; Park, S.J.; Hui, D. Generation of the pores on graphene surface and their reinforcement effects on the thermal and mechanical properties of chitosan-based composites. Compos. Part B Eng. 2017, 114, 348–355. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Lee, J.H.; Kim, H.Y.; Park, S.J. Novel magnetically separable silver-iron oxide nanoparticles decorated graphitic carbon nitride nano-sheets: A multifunctional photocatalyst via one-step hydrothermal process. J. Colloid Interface Sci. 2017, 496, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ge, X.; Li, M.-C.; Deng, F.; Oh, J.; Cho, U.R. The properties of rice bran carbon/nitrile-butadiene rubber composites fabricated by latex compounding method. Polym. Compos. 2018, 39, E687–E696. [Google Scholar] [CrossRef]
- Fan, Y.; Cho, U.R. Effects of graphite and boron nitride based fillers on mechanical, thermal conductive, and thermo-physical properties in solution styrene-butadiene rubber. Polym. Compos. 2018. [Google Scholar] [CrossRef]
- Zhang, Y.; Cho, U.R. Enhanced Interfacial Interactions of Isocyanate-Grafted Graphene Oxide/Nitrile-Butadiene Rubber Nanocomposites: Mechanical and Thermo-Physical Properties. Polym. Compos. 2018. [Google Scholar] [CrossRef]
- Gao, C.; Chen, G. Conducting polymer/carbon particle thermoelectric composites: Emerging green energy materials. Compos. Sci. Technol. 2016, 124, 52–70. [Google Scholar] [CrossRef]
- Hong, C.T.; Kang, Y.H.; Ryu, J.; Cho, S.Y.; Jang, K.S. Spray-printed CNT/P3HT organic thermoelectric films and power generators. J. Mater. Chem. A 2015, 3, 21428–21433. [Google Scholar] [CrossRef]
- Meng, B.C.; Liu, C.; Fan, S. A Promising Approach to Enhanced Thermoelectric Properties Using Carbon Nanotube Networks. Adv. Mater. 2010, 22, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, Y.; Park, S.G.; Kim, Y.G.; Sung, B.J.; Jang, S.Y.; Kim, W. Effect of the carbon nanotube type on the thermoelectric properties of CNT/Nafion nanocomposites. Org. Electron. Phys. Mater. Appl. 2011, 12, 2120–2125. [Google Scholar] [CrossRef]
- Moriarty, G.P.; Briggs, K.; Stevens, B.; Yu, C.; Grunlan, J.C. Fully Organic Nanocomposites with High Thermoelectric Power Factors by using a Dual-Stabilizer Preparation. Energy Technol. 2013, 1, 265–272. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, Q.; Wang, L.; Chen, L. Abnormally enhanced thermoelectric transport properties of SWNT/PANI hybrid films by the strengthened PANI molecular ordering. Energy Environ. Sci. 2014, 7, 3801–3807. [Google Scholar] [CrossRef]
- Lee, W.; Kang, Y.H.; Lee, J.Y.; Jang, K.S.; Cho, S.Y. Improving the thermoelectric power factor of CNT/PEDOT:PSS nanocomposite films by ethylene glycol treatment. RSC Adv. 2016, 6, 53339–53344. [Google Scholar] [CrossRef]
- Bounioux, C.; Díaz-Chao, P.; Campoy-Quiles, M.; Martín-González, M.S.; Goñi, A.R.; Yerushalmi-Rozen, R.; Müller, C. Thermoelectric composites of poly(3-hexylthiophene) and carbon nanotubes with a large power factor. Energy Environ. Sci. 2013, 6, 918–925. [Google Scholar] [CrossRef]
- Carbon, S.; Hybrid, N.P. Enhanced Thermoelectric Performance of Single-Walled Carbon Nanotubes/Polyaniline Hybrid Nanocomposites. ACS Nano 2010, 4, 2445–2451. [Google Scholar]
- Chen, Y.C.; Lee, S.C.; Liu, T.H.; Chang, C.C. Thermal conductivity of boron nitride nanoribbons: Anisotropic effects and boundary scattering. Int. J. Therm. Sci. 2015, 94, 72–78. [Google Scholar] [CrossRef]
- Wang, L.; Yao, Q.; Xiao, J.; Zeng, K.; Qu, S.; Shi, W.; Wang, Q.; Chen, L. Engineered Molecular Chain Ordering in Single-Walled Carbon Nanotubes/Polyaniline Composite Films for High-Performance Organic Thermoelectric Materials. Chem. Asian J. 2016, 11, 1804–1810. [Google Scholar] [CrossRef]
- Hu, X.; Chen, G.; Wang, X. An unusual coral-like morphology for composites of poly(3,4-ethylenedioxythiophene)/carbon nanotube and the enhanced thermoelectric performance. Compos. Sci. Technol. 2017, 144, 43–50. [Google Scholar] [CrossRef]
- Lin, Y.; Williams, T.V.; Xu, T.B.; Cao, W.; Elsayed-Ali, H.E.; Connell, J.W. Aqueous dispersions of few-layered and monolayered hexagonal boron nitride nanosheets from sonication-assisted hydrolysis: Critical role of water. J. Phys. Chem. C 2011, 115, 2679–2685. [Google Scholar] [CrossRef]
- Science, C. Thermoelectric figure of merit of a one-dimensional conductor. Phys. Rev. B 1993, 47, 8–11. [Google Scholar]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Seo, M.K.; Lee, J.R.; Park, S.J. Crystallization kinetics and interfacial behaviors of polypropylene composites reinforced with multi-walled carbon nanotubes. Mater. Sci. Eng. A 2005, 404, 79–84. [Google Scholar] [CrossRef]
- Dürkop, T.; Getty, S.A.; Cobas, E.; Fuhrer, M.S. Extraordinary Mobility in Semiconducting Carbon Nanotubes. Nano Lett. 2004, 4, 35–39. [Google Scholar] [CrossRef]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal transport measurements of individual multiwalled nanotubes. Phys. Rev. Lett. 2001, 87, 215502-1–215502-4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Choi, J.R.; Park, S.-J. Enhancing the heat and load transfer efficiency by optimizing the interface of hexagonal boron nitride/elastomer nanocomposites for thermal management applications. Polymer 2018, 143, 1–9. [Google Scholar] [CrossRef]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme Oxygen Sensitivity of Electronic Properties of Carbon Nanotubes. Science 2000, 287, 1801–1805. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Liu, B. Carbon Nanotube-Based Organic Thermoelectric Materials for Energy Harvesting. Polymers 2018, 10, 1196. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, Q.; Chang, J.; Chen, L. Enhanced thermoelectric properties of CNT/PANI composite nanofibers by highly orienting the arrangement of polymer chains. J. Mater. Chem. 2012, 22, 17612–17618. [Google Scholar] [CrossRef]
- Gao, C.; Chen, G. In Situ Oxidation Synthesis of p-Type Composite with Narrow-Bandgap Small Organic Molecule Coating on Single-Walled Carbon Nanotube: Flexible Film and Thermoelectric Performance. Small 2018, 14, e1703453. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, C.; Song, H. Improved thermoelectric performance of PEDOT: PSS films prepared by polar-solvent vapor annealing method. J. Mater. Sci. Mater. Electron. 2013, 24, 4240–4246. [Google Scholar] [CrossRef]
- Yusupov, K.; Zakhidov, A.; You, S.; Stumpf, S.; Martinez, P.M.; Ishteev, A.; Vomiero, A.; Khovaylo, V.; Schubert, U. Influence of oriented CNT forest on thermoelectric properties of polymer-based materials. J. Alloys Compd. 2018, 741, 392–397. [Google Scholar] [CrossRef]
- Hsu, J.H.; Choi, W.; Yang, G.; Yu, C. Origin of unusual thermoelectric transport behaviors in carbon nanotube filled polymer composites after solvent/acid treatments. Org. Electron. Phys. Mater. Appl. 2017, 45, 182–189. [Google Scholar] [CrossRef]
- Wu, G.; Gao, C.; Chen, G.; Wang, X.; Wang, H. High-performance organic thermoelectric modules based on flexible films of a novel n-type single-walled carbon nanotube. J. Mater. Chem. A 2016, 4, 14187–14193. [Google Scholar] [CrossRef]
- Montgomery, D.S.; Hewitt, C.A.; Barbalace, R.; Jones, T.; Carroll, D.L. Spray doping method to create a low-pro fi le high-density carbon nanotube thermoelectric generator. Carbon 2016, 96, 778–781. [Google Scholar] [CrossRef]
- Toshima, N.; Oshima, K.; Anno, H.; Nishinaka, T.; Ichikawa, S.; Iwata, A.; Shiraishi, Y. Novel Hybrid Organic Thermoelectric Materials:Three-Component Hybrid Films Consisting of a Nanoparticle Polymer Complex, Carbon Nanotubes, and Vinyl Polymer. Adv. Mater. 2015, 27, 2246–2251. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.K.; Russ, B.; Fronk, S.L.; Hu, N.; Chan-Park, M.B.; Urban, J.J.; Segalman, R.A.; Chabinyc, M.L.; Bazan, G.C. Varying the ionic functionalities of conjugated polyelectrolytes leads to both p- and n-type carbon nanotube composites for flexible thermoelectrics. Energy Environ. Sci. 2015, 8, 2341–2346. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, W.; Kang, Y.H.; Cho, S.Y.; Jang, K.S. Wet-spinning and post-treatment of CNT/PEDOT:PSS composites for use in organic fiber-based thermoelectric generators. Carbon 2018, 133, 293–299. [Google Scholar] [CrossRef]
- Wang, H.; Hsu, J.H.; Yi, S.I.; Kim, S.L.; Choi, K.; Yang, G.; Yu, C. Thermally Driven Large N-Type Voltage Responses from Hybrids of Carbon Nanotubes and Poly(3,4-ethylenedioxythiophene) with Tetrakis(dimethylamino)ethylene. Adv. Mater. 2015, 27, 6855–6861. [Google Scholar] [CrossRef]
- Xu, K.; Chen, G.; Qiu, D. Convenient construction of poly(3,4-ethylenedioxythiophene)-graphene pie-like structure with enhanced thermoelectric performance. J. Mater. Chem. A 2013, 1, 12395–12399. [Google Scholar] [CrossRef]
- Kim, G.H.; Hwang, D.H.; Woo, S.I. Thermoelectric properties of nanocomposite thin films prepared with poly(3,4-ethylenedioxythiophene) poly(styrenesulfonate) and graphene. Phys. Chem. Chem. Phys. 2012, 14, 3530–3536. [Google Scholar] [CrossRef]
- Yu, D.; Liu, F. Synthesis of carbon nanotubes by rolling up patterned graphene nanoribbons using selective atomic adsorption. Nano Lett. 2007, 7, 3046–3050. [Google Scholar] [CrossRef]
- Yoo, D.; Kim, J.; Kim, J.H. Direct synthesis of highly conductive poly(3,4-ethylenedioxythiophene):Poly(4-styrenesulfonate) (PEDOT:PSS)/graphene composites and their applications in energy harvesting systems. Nano Res. 2014, 7, 717–730. [Google Scholar] [CrossRef]
- Xiang, J.; Drzal, L.T. Templated growth of polyaniline on exfoliated graphene nanoplatelets (GNP) and its thermoelectric properties. Polymer 2012, 53, 4202–4210. [Google Scholar] [CrossRef]
- Du, Y.; Shen, S.Z.; Yang, W.; Donelson, R.; Cai, K.; Casey, P.S. Simultaneous increase in conductivity and Seebeck coefficient in a polyaniline/graphene nanosheets thermoelectric nanocomposite. Synth. Met. 2012, 161, 2688–2692. [Google Scholar] [CrossRef]
- Lu, Y.; Song, Y.; Wang, F. Thermoelectric properties of graphene nanosheets-modified polyaniline hybrid nanocomposites by an in situ chemical polymerization. Mater. Chem. Phys. 2013, 138, 238–244. [Google Scholar] [CrossRef]
- Zuev, Y.M.; Chang, W.; Kim, P. Thermoelectric and Magnetothermoelectric Transport Measurements of Graphene. Phys. Rev. Lett. 2008, 102, 096807. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, S.J. Influence of multi-walled carbon nanotubes on the electrochemical performance of graphene nanocomposites for supercapacitor electrodes. Electrochim. Acta 2011, 56, 1629–1635. [Google Scholar] [CrossRef]
- Lee, J.H.; Marroquin, J.; Rhee, K.Y.; Park, S.J.; Hui, D. Cryomilling application of graphene to improve material properties of graphene/chitosan nanocomposites. Compos. Part B Eng. 2013, 45, 682–687. [Google Scholar] [CrossRef]
- Janković, A.; Eraković, S.; Mitrić, M.; Matić, I.Z.; Juranić, Z.D.; Tsui, G.C.P.; Tang, C.Y.; Mišković-Stanković, V.; Rhee, K.Y.; Park, S.J. Bioactive hydroxyapatite/graphene composite coating and its corrosion stability in simulated body fluid. J. Alloys Compd. 2015, 624, 148–157. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. Isothermal exfoliation of graphene oxide by a new carbon dioxide pressure swing method. Carbon 2014, 68, 112–117. [Google Scholar] [CrossRef]
- Xiong, J.; Jiang, F.; Shi, H.; Xu, J.; Liu, C.; Zhou, W.; Jiang, Q.; Zhu, Z.; Hu, Y. Liquid exfoliated graphene as dopant for improving the thermoelectric power factor of conductive PEDOT:PSS nanofilm with hydrazine treatment. ACS Appl. Mater. Interfaces 2015, 7, 14917–14925. [Google Scholar] [CrossRef]
- Wang, L.; Bi, H.; Yao, Q.; Ren, D.; Qu, S.; Huang, F.; Chen, L. Three-dimensional tubular graphene/polyaniline composites as high-performance elastic thermoelectrics. Compos. Sci. Technol. 2017, 150, 135–140. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lee, T.C.; Hsiao, Y.S.; Lin, W.K.; Whang, W.T.; Chen, C.H. Facile Synthesis of Diamino-Modified Graphene/Polyaniline Semi-Interpenetrating Networks with Practical High Thermoelectric Performance. ACS Appl. Mater. Interfaces 2018, 10, 4946–4952. [Google Scholar] [CrossRef]
- Du, Y.; Li, H.; Jia, X.; Dou, Y.; Xu, J.; Eklund, P. Preparation and Thermoelectric Properties of Graphite/poly(3,4-ethyenedioxythiophene) Nanocomposites. Energies 2018, 11, 2849. [Google Scholar] [CrossRef]
- Pan, C.; Zhang, L.; Pan, Z.; Chen, M.; Liu, Y.; Huang, G.; Na, H.; Wang, W.; Qiu, H.; Gao, J. A simple strategy to fabricate polyaniline/expanded graphite composites with improved power factor. Mater. Chem. Phys. 2015, 167, 315–319. [Google Scholar] [CrossRef]
- Choi, J.; Lee, J.Y.; Lee, S.S.; Park, C.R.; Kim, H. High-Performance Thermoelectric Paper Based on Double Carrier-Filtering Processes at Nanowire Heterojunctions. Adv. Energy Mater. 2016, 6, 1502181. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Wang, L.; Du, K.; Yin, Q.; Yin, Q. Polypyrrole/graphene/polyaniline ternary nanocomposite with high thermoelectric power factor. ACS Appl. Mater. Interfaces 2017, 9, 20124–20131. [Google Scholar] [CrossRef]
- Meng, Q.; Cai, K.; Du, Y.; Chen, L. Preparation and thermoelectric properties of SWCNT/PEDOT:PSS coated tellurium nanorod composite films. J. Alloys Compd. 2019, 778, 163–169. [Google Scholar] [CrossRef]
- Cho, C.; Wallace, K.L.; Tzeng, P.; Hsu, J.H.; Yu, C.; Grunlan, J.C. Outstanding Low Temperature Thermoelectric Power Factor from Completely Organic Thin Films Enabled by Multidimensional Conjugated Nanomaterials. Adv. Energy Mater. 2016, 6, 1502168. [Google Scholar] [CrossRef]




| Materials | Chemical Structure |
|---|---|
| Polyaniline |  |
| Polypyrrole |  |
| Polythiophene |  |
| Polyacetylene |  |
| Poly(3,4-ethylenedioxythiophene) |  |
| Polyphenylenevinylene |  |
| Poly(3-methylthiophene) |  |
| Poly(2,7-Carbazolylenevinylene) |  |
| Poly(3-octylthiophene) |  |
| Poly(3-hexylthiophene) |  |
| Poly(vinylidene fluoride) |  |
| Materials | σ (S/m) | S (µV/K) | κ (W/mk) | PF (µW/mK2) | ZT | Temperature (K) |
|---|---|---|---|---|---|---|
| P3HT/CNT [125] | 345 ± 88 | 97 ± 11 | 325 ± 101 | 298 | ||
| PEDOT:PSS/SWCNT [61] | ~105 | 41 | 0.2–0.4 | ~160 | 300 | |
| PANI/CNT [126] | ~6000 | ~29 | ~5 | 298 | ||
| Nafion/MCNT [127] | 1300 | 24 | 0.07 | 0.001 | 298 | |
| PEDOT:PSS/MWNT [128] | 9500 | 40 | 0.12 | 20 | 298 | |
| PEDOT:PSS/DWNT [128] | 96,000 | 70 | 500 | 298 | ||
| PANI/SWCNT [129] | 76,900 | 65 | ~0.2 | 176 | 0.12 | 298 |
| PEDOT:PSS/CNT [130] | 78,000 ± 5100 | 43.7 ± 3.3 | 151 ± 34 | 298 | ||
| P3HT/SWCNT [131] | ~105 | ~35 | ~0.19 | 95 ± 12 | >10−2 | 298 |
| PANI/SWCNT [132] | 1.25 × 104 | 40 | 2 × 10−5 | 0.004 | 298 | |
| Diethylenetriamine/SWCNT [133] | 16,500 ± 1000 | −41.0 ± 1.5 | 298 | |||
| PANI/SWCNT [134] | 1.44 × 105 | 40 | 0.44 | 217 | 298 | |
| PEDOT/SWCNT [135] | 57,040 ± 1580 | 17.5 | 19.00 ± 1.43 | 298 |
| Materials | σ (S/m) | S (µV/K) | κ (W/mk) | PF (µW/mK2) | ZT | Temperature (K) |
|---|---|---|---|---|---|---|
| PEDOT/rGO [152] | 5000 | 31.8 | 5.2 ± 0.9 | 298 | ||
| PEDOT:PSS/graphene (100:1) [153] | 1469 | 46.9 | 0.19 | 3.23 | 0.00046 | 300 |
| PEDOT:PSS/graphene (100:2) [153] | 3200 | 59 | 0.14 | 11.09 | 0.021 | 300 |
| PEDOT:PSS/graphene (100:3) [153] | 3170 | 44.75 | 0.3 | 6.34 | 0.00057 | 300 |
| PANi/Graphene [154] | 5000 | 30 | 5.6 | 298 | ||
| PEDOT:PSS/Graphene [155] | 63,700 | 26.778 | 45.67 | 298 | ||
| PANI/GNPs [156] | 5900 | 33 | 13 | 1.51 × 10−4 | 300 | |
| PANi/Graphene pellet [157] | 5889 | 31 | 5.6 | 300 | ||
| PANi/Graphene film [157] | 863 | 41.3 | 1.47 | 300 | ||
| PANi/Graphene [158] | 2800 | 25 | 2.6 | 0.000195 | 453 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Heo, Y.-J.; Park, M.; Park, S.-J. Recent Advances in Organic Thermoelectric Materials: Principle Mechanisms and Emerging Carbon-Based Green Energy Materials. Polymers 2019, 11, 167. https://doi.org/10.3390/polym11010167
Zhang Y, Heo Y-J, Park M, Park S-J. Recent Advances in Organic Thermoelectric Materials: Principle Mechanisms and Emerging Carbon-Based Green Energy Materials. Polymers. 2019; 11(1):167. https://doi.org/10.3390/polym11010167
Chicago/Turabian StyleZhang, Yinhang, Young-Jung Heo, Mira Park, and Soo-Jin Park. 2019. "Recent Advances in Organic Thermoelectric Materials: Principle Mechanisms and Emerging Carbon-Based Green Energy Materials" Polymers 11, no. 1: 167. https://doi.org/10.3390/polym11010167
APA StyleZhang, Y., Heo, Y. -J., Park, M., & Park, S. -J. (2019). Recent Advances in Organic Thermoelectric Materials: Principle Mechanisms and Emerging Carbon-Based Green Energy Materials. Polymers, 11(1), 167. https://doi.org/10.3390/polym11010167