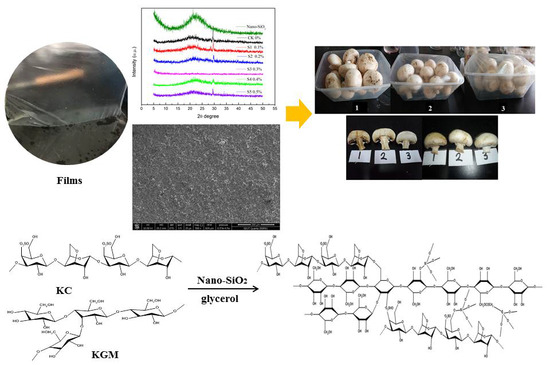
Synthesis and Characterization of Konjac Glucomannan/Carrageenan/Nano-silica Films for the Preservation of Postharvest White Mushrooms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composite Films
2.3. Characterization of Films
2.4. Properties of Films
2.4.1. Thickness
2.4.2. Water Vapor Transition Rate (WVTR)
2.4.3. Tensile Strength (Ts)
2.4.4. Color
2.4.5. Transparency
2.4.6. Water Solubility (WS) and Moisture Absorption (MA)
2.4.7. Oxygen Transmission Rate (OTR) and Carbon Dioxide Transmission Rate (CDTR)
2.5. Application of Films for Mushroom Storage Stability
2.6. Statistical Design
3. Results and Discussion
3.1. FTIR Analysis
3.2. XRD Analysis
3.3. SEM Analysis
3.4. Color and Transparency Analysis
3.5. Tensile Strength Analysis
3.6. Film Thickness Analysis
3.7. WVTR Analysis
3.8. WS and MA Analysis
3.9. OTR and CDTR Analysis
3.10. Application of Films for Mushroom Storage Stability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Persin, Z.; Stana-Kleinschek, K.; Foster, T.J.; Van Dam, J.E.G.; Boeriu, C.G.; Navard, P. Challenges and opportunities in polysaccharides research and technology: The EPNOE views for the next decade in the areas of materials, food and health care. Carbohydr. Polym. 2011, 84, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Aziz, M.S.; Salama, H.E.; Sabaa, M.W. Biobased alginate/castor oil edible films for active food packaging. LWT Food Sci. Technol. 2018, 96, 455–460. [Google Scholar] [CrossRef]
- Cheng, L.H.; Abd Karim, A.; Seow, C.C. Characterisation of composite films made of konjac glucomannan (KGM), carboxymethyl cellulose (CMC) and lipid. Food Chem. 2008, 107, 411–418. [Google Scholar] [CrossRef]
- Pang, J.; Lin, B.; Zhang, P.S.; Tian, S.P.; Sun, Y.J. Progress in the Application and Studies on Functional Material of Konjac Glucomannan. Chin. J. Struct. Chem. 2003, 22, 633–642. [Google Scholar]
- Thành, T.T.T.; Yuguchi, Y.; Mimura, M.; Yasunaga, H.; Takano, R.; Urakawa, H. Molecular Characteristics and Gelling Properties of the Carrageenan Family, 1. Preparation of Novel Carrageenans and their Dilute Solution Properties. Macromol. Chem. Phys. 2002, 203, 15–23. [Google Scholar] [CrossRef]
- Johnston, J.H.; Grindrod, J.E.; Dodds, M.; Schimitschek, K.C. Composite nano-structured calcium silicate phase change materials for thermal buffering in food packaging. Appl. Phys. 2008, 8, 508–511. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; Bhandari, B.; He, Y.H. Nano-particle encapsulation of fish oil by spray drying. Food Res. Int. 2008, 41, 172–183. [Google Scholar] [CrossRef]
- Taira, S.; Sahashi, Y. Nanoparticle-assisted laser desorption/ionization (nano-PALDI) mass spectrometry for food analysis. J. Biosci. Bioeng. 2009, 108, S155. [Google Scholar] [CrossRef]
- Hu, G.H.; Hoppe, S.; Feng, F.; Fonteix, C. Nano-scale phenomena and applications in polymer processing. Chem. Eng. Sci. 2007, 62, 3528–3537. [Google Scholar] [CrossRef]
- Rhim, J.W.J.; Lee, S.B.; Hong, S.I. Preparation and characterization of agar/clay nanocomposite films: The effect of clay type. Food Sci. 2011, 76, N40–N48. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wu, C.L.; Hao, H.; Dai, Y.; Li, J.R. Preparation and preservation properties of the chitosan coatings modified with the in situ, synthesized nano SiOx. Food Hydrocolloid. 2016, 54, 130–138. [Google Scholar] [CrossRef]
- Ahmadizadegan, H. Surface modification of TiO2 nanoparticles with biodegradable nanocellolose and synthesis of novel polyimide/cellulose/TiO2 membrane. J. Colloid Interfaces Sci. 2017, 491, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, A.; Khoylou, F.; Ataeivarjovi, E. Preparation and characterization of gamma irradiated Starch/PVA/ZnO nanocomposite films. Radiat. Phys. Chem. 2017, 138, 49–53. [Google Scholar] [CrossRef]
- U.S. Food and Drug Adminastration. Food Additive Status List; U.S. Food and Drug Adminastration: Silver Spring, MD, USA, 2011.
- Abbasi, Z. Water resistance, weight loss and enzymatic degradation of blends starch/polyvinyl alcohol containing SiO2 nanoparticle. J. Taiwan Chem. Eng. 2012, 43, 264–268. [Google Scholar] [CrossRef]
- Lei, Z.L.; Bi, S.X.; Yang, H. Chitosan-tethered the silica particle from a layer-by-layer approach for pectinase immobilization. Food Chem. 2007, 104, 577–584. [Google Scholar] [CrossRef]
- Yan, J.; Li, J.; Zhao, H.; Chen, N.; Cao, J.; Jiang, W.J. Effects of oligo chitosan on postharvest Alternaria rot, storage quality, and defense responses in Chinese jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit. Food Prot. 2011, 74, 783–788. [Google Scholar] [CrossRef]
- Zhang, R.F.; Wang, X.Y.; Li, L.; Cheng, M.; Zhang, L.M. Optimization of konjac glucomannan/carrageenan/nano-SiO2 coatings for extending the shelf-life of Agaricus bisporus. Int. J. Biol. Macromol. 2019, 122, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Peng, S.H.; Wen, C.R.; Wang, X.M.; Fan, L.L.; Deng, R.H.; Pang, J. Structural characterization and properties of konjac glucomannan/curdlan blend films. Carbohydr. Polym. 2012, 89, 497–503. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, M.; Dai, S.H.; Song, J.; Ni, X.W.; Fang, Y.P.; Corke, H.; Jiang, F.T. Interactions between carboxymethyl konjac glucomannan and soy protein isolate in blended films. Carbohydr. Polym. 2014, 101, 136–145. [Google Scholar] [CrossRef]
- Zhu, G.L.; Sheng, L.; Tong, Q.Y. Preparation and characterization of carboxymethyl-gellan and pullulan blend films. Food Hydrocoll. 2014, 35, 341–347. [Google Scholar] [CrossRef]
- Hassannia-Kolaee, M.; Khodaiyan, F.; Pourahmad, R.; Ghahfarrokhi, I.S. Development of ecofriendly bionanocomposite: Whey protein isolate/pullulan films with nano-SiO2. Int. J. Biol. Macromol. 2016, 86, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.W.; Hong, S.I.; Park, H.M.; Ng, P.K.W. Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 5814–5822. [Google Scholar] [CrossRef] [PubMed]
- Moreno, O.; Cárdenas, J.; Atarés, L.; Chiralt, A. Influence of starch oxidation on the functionality of starch-gelatin based active films. Carbohydr. Polym. 2017, 178, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Wang, X.Y.; Zhu, J.Y.; Wang, J. Effect of high oxygen modified atmosphere on post-harvest physiology and sensorial qualities of mushroom. Int. J. Food Sci. Technol. 2010, 45, 1097–1103. [Google Scholar] [CrossRef]
- Liu, J.; Lv, Y.; Mo, X.; Duan, S.; Tong, Q. Effects of freezing and thawing treatment on the rheological and textural characteristics and micro-structure of heat-induced egg yolk gels. J. Food Eng. 2018, 216, 144–150. [Google Scholar] [CrossRef]
- Li, T.; Guo, X.; Zhu, K.; Zhou, H. Effects of alkali on protein polymerization and textural characteristics of textured wheat protein. Food Chem. 2018, 239, 579–587. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, H.B.; Yin, Y.M.; Nishinari, K. Comparison of curdlan and its carboxymethylated derivative by means of Rheology, DSC, and AFM. Carbohydr. Res. 2006, 341, 90–99. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Li, Y.Z.; Lv, M.Z.; Li, P.W.; Xu, H.L.; Wang, L. Preparation and characterization of novel curdlan/chitosan blending membranes for antibacterial applications. Carbohydr. Polym. 2011, 84, 952–959. [Google Scholar] [CrossRef]
- Yao, K.; Caia, J.; Liu, M. Structure and properties of starch/PVA/nano-silica hybrid films. Carbohydr. Polym. 2011, 86, 1784–1789. [Google Scholar] [CrossRef]
- Tong, Q.; Xiao, Q.; Lim, L.T. Preparation and properties of pullulan-alginate-carboxymethylcellulose blend films. Food Res. Int. 2008, 41, 1007–1014. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, F.; Li, Y.; Shoemaker, C.F.; Xia, W. Preparation and characterization of pullulan–chitosan and pullulan–carboxymethyl chitosan blended films. Food Hydrocoll. 2013, 30, 82–91. [Google Scholar] [CrossRef]
- Abdollahi, M.; Alboofetileh, M.; Rezaei, M.; Behrooz, R. Comparing physico-mechanical and thermal properties of alginate nanocomposite films reinforced with organic and/or inorganic nanofillers. Food Hydrocoll. 2013, 32, 416–424. [Google Scholar] [CrossRef]
- Lizundia, E.; Vilas, J.L.; Sangroniz, A.; Etxeberria, A. Light and gas barrier properties of PLLA/metallic nanoparticles composite films. Eur. Polym. J. 2017, 91, 10–20. [Google Scholar] [CrossRef]
- Chaichana, E.; Jongsomjit, B.; Praserthdam, P. Effect of nano-SiO2, particle size on the formation of LLDPE/SiO2, nanocomposite synthesized via the in situ polymerization with metallocene catalyst. Chem. Eng. Sci. 2007, 62, 899–905. [Google Scholar] [CrossRef]
- Xiong, H.G.; Tang, S.W.; Tang, H.W. The structure and properties of a starch-based biodegradable film. Carbohydr. Polym. 2008, 71, 263–268. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S.; Cuq, J.L. Water and Glycerol as Plasticizers Affect Mechanical and Water Vapor Barrier Properties of an Edible Wheat Gluten Film. J. Food Sci. 1993, 58, 206–211. [Google Scholar] [CrossRef]
- Guerrero, P.; Hanani, Z.A.N.; Kerry, J.P.; Caba, K.D.L. Characterization of soy protein-based films prepared with acids and oils by compression. J. Food Eng. 2011, 107, 41–49. [Google Scholar] [CrossRef]
- Tabatabaei, R.H.; Jafari, S.M.; Mirzaei, H.; Nafchi, A.M.; Dehnad, D. Preparation and characterization of nano-SiO2 reinforced gelatin-k-carrageenan biocomposites. Int. J. Biol. Macromol. 2018, 111, 1091–1099. [Google Scholar] [CrossRef] [PubMed]







| Films | ∆E | T600nm (%) | Ts (MPa) |
|---|---|---|---|
| CK | 4.02 ± 0.05 a | 90.36 ± 0.35 a | 50.63 ± 2.1 f |
| S1 | 3.81 ± 0.21 ab | 83.21 ± 0.24 b | 61.32 ± 1.3 e |
| S2 | 3.32 ± 0.02 c | 80.13 ± 0.15 c | 63.51 ± 3.2 d |
| S3 | 2.67 ± 0.22 f | 73.91 ± 0.17 f | 69.11 ± 1.5 a |
| S4 | 2.98 ± 0.01 e | 74.45 ± 0.21 e | 67.22 ± 1.1 c |
| S5 | 3.19 ± 0.13 d | 75.32 ± 0.09 d | 68.01 ± 2.7 b |
| Films | Thickness (μm) | WVP (g·m−2·d−1) | WS (%) | MA (%) | OTR (g·m−2·d−1 | CDTR (g·m−2·d−1) |
|---|---|---|---|---|---|---|
| CK | 26.4 ± 0.48 a | 806.4 ± 9.12 a | 80.1 ± 0.71 a | 176.4 ± 5.88 f | 0.039 ± 0.002 f | 0.238 ± 0.011 f |
| S1 | 25.9 ± 0.31 a | 696.3 ± 5.22 d | 68.7 ± 0.85 b | 213.1 ± 6.17 e | 0.030 ± 0.001 e | 0.204 ± 0.016 cd |
| S2 | 26.3 ± 0.26 a | 662.0 ± 3.78 e | 61.3 ± 0.32 c | 271.2 ± 4.12 d | 0.023 ± 0.003 b | 0.198 ± 0.008 bc |
| S3 | 25.6 ± 0.33 a | 644.4 ± 1.44 f | 52.4 ± 0.41 f | 297.3 ± 3.25 a | 0.016 ± 0.002 a | 0.153 ± 0.021 a |
| S4 | 26.9 ± 0.21 a | 743.6 ± 2.32 c | 59.2 ± 0.56 cd | 280.5 ± 1.36 c | 0.026 ± 0.001 c | 0.187 ± 0.009 b |
| S5 | 26.2 ± 0.39 a | 783.8 ± 4.26 b | 58.4 ± 0.38 de | 286.1 ± 2.47 b | 0.028 ± 0.002 cd | 0.206 ± 0.006 de |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Wang, X.; Wang, J.; Cheng, M. Synthesis and Characterization of Konjac Glucomannan/Carrageenan/Nano-silica Films for the Preservation of Postharvest White Mushrooms. Polymers 2019, 11, 6. https://doi.org/10.3390/polym11010006
Zhang R, Wang X, Wang J, Cheng M. Synthesis and Characterization of Konjac Glucomannan/Carrageenan/Nano-silica Films for the Preservation of Postharvest White Mushrooms. Polymers. 2019; 11(1):6. https://doi.org/10.3390/polym11010006
Chicago/Turabian StyleZhang, Rongfei, Xiangyou Wang, Juan Wang, and Meng Cheng. 2019. "Synthesis and Characterization of Konjac Glucomannan/Carrageenan/Nano-silica Films for the Preservation of Postharvest White Mushrooms" Polymers 11, no. 1: 6. https://doi.org/10.3390/polym11010006
APA StyleZhang, R., Wang, X., Wang, J., & Cheng, M. (2019). Synthesis and Characterization of Konjac Glucomannan/Carrageenan/Nano-silica Films for the Preservation of Postharvest White Mushrooms. Polymers, 11(1), 6. https://doi.org/10.3390/polym11010006