Anti-Oxidant and Anti-Diabetes Potential of Water-Soluble Chitosan–Glucose Derivatives Produced by Maillard Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
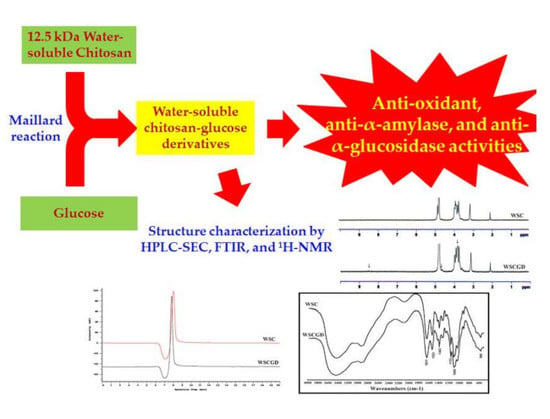
2.2. Synthesis of Water-Soluble Chitosan–Glucose Derivatives
2.3. Anti-Oxidant Activity Assay
2.4. Anti-α-Amylase Activity Assay
2.5. Anti-α-Glucosidase Activity Assay
2.6. High Performance Liquid Chromatography Size Exclusion Chromatography (HPLC SEC) Analysis
2.7. Colloid Titration Analysis
2.8. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.9. Proton Nuclear Magnetic Resonance (1H-NMR) Analysis
3. Results
3.1. Water-Soluble Chitosan–Glucose Derivatives’ (WSCGDs) Formation via Maillard Reaction
3.2. Anti-Oxidant Activity
3.3. Anti-α-Amylase Activity
3.4. Anti-α-Glucosidase Activity
3.5. HPLC SEC Analysis
3.6. Colloid Titration Analysis
3.7. FTIR Analysis
3.8. H-NMR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Wang, C.H.; Doan, C.T.; Nguyen, A.D.; Nguyen, V.B.; Wang, S.L. Reclamation of fishery processing waste: A mini-review. Molecules 2019, 24, 2234. [Google Scholar] [CrossRef] [PubMed]
- Akca, G.; Özdemir, A.; Öner, Z.G.; Şenel, S. Comparison of different types and sources of chitosan for the treatment of infections in the oral cavity. Res. Chem. Intermed. 2018, 44, 4811–4825. [Google Scholar] [CrossRef]
- Wang, S.L.; Liang, T.W. Microbial reclamation of squid pens and shrimp shell. Res. Chem. Intermed. 2017, 43, 3445–3462. [Google Scholar] [CrossRef]
- Tran, T.N.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. The isolation of chitinase from Streptomyces thermocarboxydus and its application in the preparation of chitin oligomers. Res. Chem. Intermed. 2019, 45, 727–742. [Google Scholar] [CrossRef]
- Hiranpattanakul, P.; Jongjitpissamai, T.; Aungwerojanawit, S.; Tachaboonyakiat, W. Fabrication of a chitin/chitosan hydrocolloid wound dressing and evaluation of its bioactive properties. Res. Chem. Intermed. 2018, 44, 4913–4928. [Google Scholar] [CrossRef]
- Wang, S.L.; Yu, H.T.; Tsai, M.H.; Doan, C.T.; Nguyen, V.B.; Do, V.C.; Nguyen, A.D. Conversion of squid pens to chitosanases and dye adsorbents via Bacillus cereus. Res. Chem. Intermed. 2018, 44, 4903–4911. [Google Scholar] [CrossRef]
- Ding, F.; Li, H.; Du, Y.; Shi, X. Recent advances in chitosan-based self-healing materials. Res. Chem. Intermed. 2018, 44, 4827–4840. [Google Scholar] [CrossRef]
- Mohandas, A.; Sun, W.; Nimal, T.R.; Shankarappa, S.A.; Hwang, N.S. Injectable chitosan-fibrin/nanocurcumin composite hydrogel for the enhancement of angiogenesis. Res. Chem. Intermed. 2018, 44, 4873–4887. [Google Scholar] [CrossRef]
- Jaworska, M.M.; Górak, A. New ionic liquids for modification of chitin particles. Res. Chem. Intermed. 2018, 44, 4841–4854. [Google Scholar] [CrossRef] [Green Version]
- Chouljenko, A.; Chotiko, A.; Reyes, V.; Alfaro, L.; Liu, C.; Dzandu, B.; Sathivel, S. Application of water-soluble chitosan to shrimp for quality retention. LWT 2016, 74, 571–579. [Google Scholar] [CrossRef]
- Kahya, N. Water soluble chitosan derivatives and their biological activities: A review. Polym. Sci. 2018, 4, 16. [Google Scholar]
- Yusharani, M.S.; Ulfin, I.; Lailun, Y. Synthesis of water-soluble chitosan from squid pens waste as raw material for capsule shell: Temperature deacetylation and reaction time. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012070. [Google Scholar] [CrossRef]
- Du, D.X.; Vuong, B.X. Study on preparation of water-soluble chitosan with varying molecular weights and its antioxidant activity. Adv. Mater. Sci. Eng. 2019, 2019, 8781013. [Google Scholar] [CrossRef]
- Gullón, B.; Montenegro, M.I.; Ruiz-Matute, A.I.; Cardelle-Cobas, A.; Corzo, N.; Pintado, M.E. Synthesis, optimization and structural characterization of a chitosan–glucose derivative obtained by the Maillard reaction. Carbohydr. Polym. 2016, 137, 382–389. [Google Scholar] [CrossRef]
- Mature, A.I.R.; Cardelle-Cobas, A.; García-Bermejo, A.B. Synthesis, characterization and functional properties of galactosylated derivatives of chitosan through amide formation. Food Hydrocoll. 2013, 33, 245–255. [Google Scholar] [Green Version]
- Phisut, N.; Jiraporn, B. Characteristics and antioxidant activity of Maillard reaction products derived from chitosan-sugar solution. Int. Food Res. J. 2013, 20, 1077–1085. [Google Scholar]
- Li, J.; Ma, F.K.; Dang, Q.F.; Liang, X.G.; Chen, X.G. Glucose-conjugated chitosan nanoparticles for targeted drug delivery and their specific interaction with tumor cells. Front. Mater. Sci. 2014, 8, 363–372. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Sharma, A. Chitosan glucose complex—A novel food preservative. Food Chem. 2008, 106, 521–528. [Google Scholar] [CrossRef]
- Weerakkody, R.; Labbett, D.; Cheng, L.; Kosaraju, S.L. Effect of physicochemical modifications on antioxidant activity of water-soluble chitosan. Food Biophys. 2011, 6, 127–132. [Google Scholar] [CrossRef]
- Chung, Y.C.; Tsai, C.F.; Li, C.F. Preparation and characterization of water-soluble chitosan produced by Maillard reaction. Fish. Sci. 2006, 72, 1096–1103. [Google Scholar] [CrossRef]
- Kosaraju, S.L.; Weerakkody, R.; Augustin, M.A. Chitosan-glucose conjugates: Influence of extent of Maillard reaction on antioxidant properties. J. Agric. Food Chem. 2010, 58, 12449–12455. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Qin, Y.; Xie, J.; Xue, B.; Zhu, Y.; Wu, J.; Bian, X.; Li, X. Antioxidant activity of oligochitosan Maillard reaction products using oligochitosan as the amino or carbonyl groups donors. Int. J. Food Prop. 2018, 21, 1964–1971. [Google Scholar] [CrossRef] [Green Version]
- Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Hong, J.T.; Hwang, B.Y.; Jung, J.K.; Lee, J.; Jeong, H.S. Isolation and characterisation of an α-glucosidase inhibitory substance from fructose-tyrosine Maillard reaction products. Food Chem. 2011, 127, 122–126. [Google Scholar] [CrossRef]
- Malunga, L.N.; Eck, P.; Beta, T. Inhibition of intestinal α-glucosidase and glucose absorption by feruloylated arabinoxylan mono- and oligosaccharides from corn bran and wheat aleurone. J. Nutr. Metab. 2016, 2016, 1932532. [Google Scholar] [CrossRef]
- Sheliya, M.A.; Begum, R.; Pillai, K.K.; Aeri, V.; Mir, S.R.; Ali, A.; Sharma, M. In vitro α-glucosidase and α-amylase inhibition by aqueous, hydroalcoholic, and alcoholic extract of Euphorbia hirta L. Drug Dev. Ther. 2016, 7, 26–30. [Google Scholar]
- Sun, H.; Song, X.; Tao, Y.; Li, M.; Yang, K.; Zheng, H.; Jin, Z.; Dodd, R.H.; Pan, G.; Lu, K.; et al. Synthesis & α-glucosidase inhibitory & glucose consumption-promoting activities of flavonoid-coumarin hybrids. Future Med. Chem. 2018, 10, 1055–1066. [Google Scholar]
- Huang, T.T.; Wang, S.L.; Nguyen, V.B.; Kuo, Y.H. Isolation and identification of potent antidiabetic compounds from Antrodia cinnamomea—An edible Taiwanese mushroom. Molecules 2018, 23, 2864. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Wang, S.L.; Nguyen, T.H.; Nguyen, M.T.; Doan, C.T.; Tran, T.N.; Lin, Z.H.; Nguyen, Q.V.; Kuo, Y.H.; Nguyen, A.D. Novel potent hypoglycemic compounds from Euonymus laxiflorus Champ. and their effect on reducing plasma glucose in an ICR mouse model. Molecules 2018, 23, 1928. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Wang, S.L.; Ngu, T.N.; Nguyen, T.H.; Nguyen, P.D.N.; Do, H.N.; Nguyen, M.C. New records of potent in-vitro antidiabetic properties of Dalbergia tonkinensis heartwood and the bioactivity-guided isolation of active compounds. Molecules 2018, 23, 1589. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Wang, S.L. New novel α–glucosidase inhibitors produced by microbial conversion. Process Biochem. 2018, 65, 228–232. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, T.H.; Doan, C.T.; Tran, T.N.; Nguyen, A.D.; Kuo, Y.H.; Wang, S.L. Production and bioactivity-guided isolation of antioxidants with α-glucosidase inhibitory and anti-NO properties from marine chitinous material. Molecules 2018, 23, 1124. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.V.; Wang, S.L.; Nguyen, A.D. In vitro α-glucosidase and α-amylase inhibition, and in vivo anti-hyperglycemic effects of Psidium littorale Raddi leaf extract. Res. Chem. Intermed. 2018, 44, 1745–1753. [Google Scholar] [CrossRef]
- Sulistiyani, S.; Safithri, M.; Sari, Y.P. Inhibition of α-glucosidase activity by ethanolic extract of Melia azedarach L. leaves. IOP Conf. Ser. Earth Environ. Sci. 2016, 31, 012025. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Wang, S.L. Production of potent antidiabetic compounds from shrimp head powder via Paenibacillus conversion. Process Biochem. 2019, 76, 18–24. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Conversion of squid pens to chitosanases and proteases via Paenibacillus sp. TKU042. Mar. Drugs 2018, 16, 83. [Google Scholar] [CrossRef]
- Wang, S.L.; Su, Y.C.; Nguyen, V.B.; Nguyen, A.D. Reclamation of shrimp heads for the production of α-glucosidase inhibitors by Staphylococcus sp. TKU043. Res. Chem. Intermed. 2018, 44, 4929–4937. [Google Scholar] [CrossRef]
- Hsu, C.H.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Conversion of shrimp heads to α-glucosidase inhibitors via co-culture of Bacillus mycoides TKU040 and Rhizobium sp. TKU041. Res. Chem. Intermed. 2018, 44, 4597–4607. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Utilization of fishery processing byproduct squid pens for Paenibacillus sp. fermentation on producing potent α-glucosidase inhibitors. Mar. Drugs 2017, 15, 274. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, M.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Anti-α-glucosidase activity by a protease from Bacillus licheniformis. Molecules 2019, 24, 691. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Production of a thermostable chitosanase from shrimp heads via Paenibacillus mucilaginosus TKU032 conversion and its application in the preparation of bioactive chitosan oligosaccharides. Mar. Drugs 2019, 17, 217. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.H.; Ha, K.S.; Moon, K.S.; Kim, J.G.; Oh, C.G.; Kim, Y.C.; Apostolidis, E.; Kwon, Y.I. Molecular weight dependent glucose lowering effect of low molecular weight chitosan oligosaccharide (GO2KA1) on postprandial blood glucose level in SD rats model. Int. J. Mol. Sci. 2013, 14, 14214–14224. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, J.F.; Kan, J.; Jin, C.H. Synthesis of chitosan-gallic acid conjugate: Structure characterization and in vitro anti-diabetic potential. Int. J. Biol. Macromol. 2013, 62, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Doan, C.T.; Nguyen, M.T.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.L. An exochitinase with N-acetyl-β-glucosamine-like activity from shrimp head conversion by Streptomyces speibonae and its application in hydrolyzing β-chitin powder to produce N-acetyl-D-glucosamine. Polymers 2019, 11, 1600. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.B.; Nguyen, Q.V.; Nguyen, A.D.; Wang, S.L. Screening and evaluation of α-glucosidase inhibitors from indigenous medicinal plants in Dak Lak Province, Vietnam. Res. Chem. Intermed. 2016, 43, 3599–3612. [Google Scholar] [CrossRef]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Khan, T.M.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Ying, G.; Xiong, W.; Wang, H.; Sun, Y.; Liu, H. Preparation, water solubility and antioxidant activity of branched-chain chitosan derivatives. Carbonhyd. Polym. 2011, 83, 1787–1796. [Google Scholar] [CrossRef]
- Bakry, A.M.; Ma, C.; Xiong, S.; Yin, T.; Zhang, B.; Huang, Q. Chitosan-glucose Maillard reaction products and their preservative effects on fresh grass carp (Ctenopharyngodon idellus) fillets during cold storage. J. Sci. Food. Agric. 2019, 99, 2158–2164. [Google Scholar] [CrossRef]





| Treatment | A294 | A420 | A294/A420 |
|---|---|---|---|
| Control | 0.037 ± 0.001 g | 0.013 ± 0.001 g | 2.977 ± 0.363 e |
| 100 °C, 1 h | 0.056 ± 0.059 fg | 0.015 ± 0.010 fg | 3.763 ± 0.415 d |
| 100 °C, 2 h | 0.078 ± 0.003 f | 0.018 ± 0.001 f | 4.254 ± 0.083 d |
| 100 °C, 3 h | 0.103 ± 0.002 e | 0.024 ± 0.000 e | 4.278 ± 0.087 cd |
| 100 °C, 4 h | 0.109 ± 0.010 e | 0.025 ± 0.001 e | 4.420 ± 0.072 cd |
| 121 °C, 1 h | 0.215 ± 0.055 d | 0.032 ± 0.001 d | 6.737 ± 0.372 a |
| 121 °C, 2 h | 0.393 ± 0.089 c | 0.073 ± 0.015 c | 5.404 ± 0.089 b |
| 121 °C, 3 h | 0.598 ± 0.083 b | 0.121 ± 0.003 b | 4.932 ± 0.057 bc |
| 121 °C, 4 h | 0.744 ± 0.017 a | 0.170 ± 0.036 a | 4.379 ± 0.167 cd |
| Treatment | IC50 (mg/mL) | Maximum Activity * (%) |
|---|---|---|
| Control | ND | 25.44 ± 1.39 e |
| 100 °C, 1 h | 13.94 ± 0.75 a | 45.14 ± 3.12 d |
| 100 °C, 2 h | 9.69 ± 0.56 b | 50.71 ± 1.25 cd |
| 100 °C, 3 h | 8.75 ± 0.75 b | 52.37 ± 1.39 c |
| 100 °C, 4 h | 8.38 ± 0.72 b | 55.11 ± 1.63 c |
| 121 °C, 1 h | 4.99 ± 0.33 c | 68.33 ± 2.38 b |
| 121 °C, 2 h | 1.90 ± 0.21 d | 87.03 ± 1.94 a |
| 121 °C, 3 h | 1.28 ± 0.16 d | 92.52 ± 4.11 a |
| 121 °C, 4 h | 1.05 ± 0.19 d | 92.69 ± 1.23 a |
| Treatment | IC50 (mg/mL) | Maximum Activity * (%) |
|---|---|---|
| Control | ND | 23.13 ± 3.64 c |
| 100 °C, 1 h | ND | 25.71 ± 4.06 bc |
| 100 °C, 2 h | ND | 25.62 ± 4.78 bc |
| 100 °C, 3 h | ND | 38.25 ± 4.39 bc |
| 100 °C, 4 h | ND | 27.13 ± 6.79 bc |
| 121 °C, 1 h | ND | 31.43 ± 6.23 bc |
| 121 °C, 2 h | ND | 36.79 ± 4.67 b |
| 121 °C, 3 h | 18.02 ± 0.88 | 56.56 ± 4.51 a |
| 121 °C, 4 h | 18.37 ± 1.33 | 56.07 ± 5.67 a |
| Treatment | IC50 (mg/mL) | Maximum Activity * (%) |
|---|---|---|
| Control | 10.04 ± 0.45 a | 69.07 ± 2.04 d |
| 100 °C, 1 h | 10.17 ± 0.64 a | 70.73 ± 3.45 d |
| 100 °C, 2 h | 9.33 ± 0.07 ab | 72.02 ± 2.56 cd |
| 100 °C, 3 h | 9.13 ± 0.39 ab | 74.28 ± 2.64 cd |
| 100 °C, 4 h | 8.71 ± 0.39 b | 72.92 ± 0.87 bcd |
| 121 °C, 1 h | 7.09 ± 0.14 c | 77.42 ± 1.87 bc |
| 121 °C, 2 h | 6.15 ± 0.33 cd | 80.65 ± 2.17 b |
| 121 °C, 3 h | 5.72 ± 0.36 d | 89.16 ± 2.52 a |
| 121 °C, 4 h | 5.85 ± 0.33 d | 90.63 ± 0.56 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.N.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.-L. Anti-Oxidant and Anti-Diabetes Potential of Water-Soluble Chitosan–Glucose Derivatives Produced by Maillard Reaction. Polymers 2019, 11, 1714. https://doi.org/10.3390/polym11101714
Tran TN, Doan CT, Nguyen VB, Nguyen AD, Wang S-L. Anti-Oxidant and Anti-Diabetes Potential of Water-Soluble Chitosan–Glucose Derivatives Produced by Maillard Reaction. Polymers. 2019; 11(10):1714. https://doi.org/10.3390/polym11101714
Chicago/Turabian StyleTran, Thi Ngoc, Chien Thang Doan, Van Bon Nguyen, Anh Dzung Nguyen, and San-Lang Wang. 2019. "Anti-Oxidant and Anti-Diabetes Potential of Water-Soluble Chitosan–Glucose Derivatives Produced by Maillard Reaction" Polymers 11, no. 10: 1714. https://doi.org/10.3390/polym11101714
APA StyleTran, T. N., Doan, C. T., Nguyen, V. B., Nguyen, A. D., & Wang, S. -L. (2019). Anti-Oxidant and Anti-Diabetes Potential of Water-Soluble Chitosan–Glucose Derivatives Produced by Maillard Reaction. Polymers, 11(10), 1714. https://doi.org/10.3390/polym11101714