Development of Nontoxic Biodegradable Polyurethanes Based on Polyhydroxyalkanoate and L-lysine Diisocyanate with Improved Mechanical Properties as New Elastomers Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of LPH Films
2.3. Characterization
2.4. Contact Angle
2.5. Swelling Properties
2.6. Cell Viability Test
2.7. Statistical Analysis
3. Results and Discussions
3.1. Structural Analysis
3.2. Thermal Properties and Crystallinity Analysis
3.3. Mechanical Properties of LPH Films
3.4. Swelling Properties of LPH Films
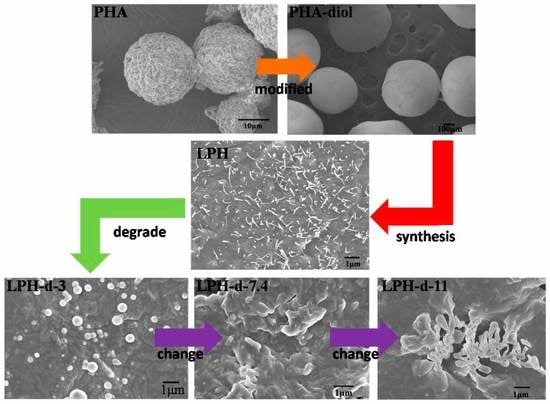
3.5. In Vitro Degradation of LPH Films
3.6. Cytocompatibility of the LPH Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kutikov, A.B.; Song, J. Biodegradable PEG-Based Amphiphilic Block Copolymers for Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2015, 1, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Sant, V.; Phillippi, J.; Sant, S. Biodegradable and biomimetic elastomeric scaffolds for tissue-engineered heart valves. Acta Biomater. 2017, 48, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Nakhoda, H.M.; Dahman, Y. Mechanical properties and biodegradability of porous polyurethanes reinforced with green nanofibers for applications in tissue engineering. Polym. Bull. 2016, 73, 2039–2055. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, L.; Pu, X. Structure, properties and biodegradability of water resistant regenerated cellulose films coated with polyurethane/benzyl konjac glucomannan semi-IPN coating. Polym. Degrad. Stab. 2004, 86, 51–57. [Google Scholar] [CrossRef]
- Atiqah, A.; Mastura, M.T.; Ahmed Ali, B.A.; Jawaid, M.; Sapuan, S.M. A Review on Polyurethane and its Polymer Composites. Curr. Org. Synth. 2017, 14, 233–248. [Google Scholar] [CrossRef]
- Hu, J.L.; Mondal, S. Structural characterization and mass transfer properties of segmented polyurethane: Influence of block length of hydrophilic segments. Polym. Int. 2010, 54, 764–771. [Google Scholar] [CrossRef]
- Choi, T.; Weksler, J.; Padsalgikar, A.; Runt, J. Influence of soft segment composition on phase-separated microstructure of polydimethylsiloxane-based segmented polyurethane copolymers. Polymer 2009, 50, 2320–2327. [Google Scholar] [CrossRef]
- Zachman, A.L.; Page, J.M.; Prabhakar, G.; Guelcher, S.A.; Sung, H.J. Elucidation of adhesion-dependent spontaneous apoptosis in macrophages using phase separated PEG/polyurethane films. Acta Biomater. 2013, 9, 4964–4975. [Google Scholar] [CrossRef]
- Kojio, K.; Nakamura, S.; Furukawa, M. Effect of side groups of polymer glycol on microphase-separated structure and mechanical properties of polyurethane elastomers. J. Polym. Sci. Part B Polym. Phys. 2010, 46, 2054–2063. [Google Scholar] [CrossRef]
- Yong, H.; Xie, D.; Zhang, X. The structure, microphase-separated morphology, and property of polyurethanes and polyureas. J. Mater. Sci. 2014, 49, 7339–7352. [Google Scholar]
- Singh, H.; Sharma, T.P.; Jain, A.K. Reactivity of the raw materials and their effects on the structure and properties of rigid polyurethane foams. J. Appl. Polym. Sci. 2010, 106, 1014–1023. [Google Scholar] [CrossRef]
- Chalid, M.; Heeres, H.J.; Broekhuis, A.A. A Study on the Structure of Novel Polyurethanes Derived from γ-Valerolactone-Based Diol Precursors. Adv. Mater. Res. 2013, 789, 274–278. [Google Scholar] [CrossRef]
- Cao, H.; Kuboyama, N. A biodegradable porous composite scaffold of PGA/beta-TCP for bone tissue engineering. Bone 2010, 46, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Causa, F.; Taddei, P.; Di, F.M.; Ciapetti, G.; Martini, D.; Fagnano, C.; Baldini, N.; Ambrosio, L. Polylactic acid fibre-reinforced polycaprolactone scaffolds for bone tissue engineering. Biomaterials 2008, 29, 3662–3670. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, X.S. Mechanical and Thermal Properties of Biocomposites from Poly(lactic acid) and DDGS. J. Appl. Polym. Sci. 2011, 121, 589–597. [Google Scholar] [CrossRef]
- Williamson, M.R.; Adams, E.F.; Coombes, A.G. Gravity spun polycaprolactone fibres for soft tissue engineering: Interaction with fibroblasts and myoblasts in cell culture. Biomaterials 2006, 27, 1019–1026. [Google Scholar] [CrossRef]
- Pereira, I.H.; Ayres, E.; Patricio, P.S.; Goes, A.M.; Gomide, V.S.; Junior, E.P.; Orefice, R.L. Photopolymerizable and injectable polyurethanes for biomedical applications: Synthesis and biocompatibility. Acta Biomater. 2010, 6, 3056–3066. [Google Scholar] [CrossRef]
- Pooja, B.; Barbara, L.; Rinat, N.; Ipsita, R. Development of novel scl-mcl polyhydroxyalkanoates blends for soft tissue engineering. Front. Bioeng. Biotechnol. 2016, 4, 1–2. [Google Scholar] [CrossRef]
- Liu, W.J.; Xue, Q.L.; Wang, S.F. Strategies of polyhydroxyalkanoates modification for the medical application in neural regeneration/nerve tissue engineering. Adv. Biosci. Biotechnol. 2013, 4, 731–740. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Hsieh, C.-T.; Sun, Y.-M. Synthesis and characterization of waterborne polyurethane containing poly(3-hydroxybutyrate) as new biodegradable elastomers. J. Mater. Chem. B 2015, 3, 9089–9097. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peponi, L. Polyurethane based on PLA and PCL incorporated with catechin: Structural, thermal and mechanical characterization. Eur. Polym. J. 2017, 89, 174–184. [Google Scholar] [CrossRef]
- Wiles, D.M.; Scott, G. Polyolefins with controlled environmental degradability. Polym. Degrad. Stab. 2006, 91, 1581–1592. [Google Scholar] [CrossRef]
- Wang, Y.; Rudym, D.D.; Walsh, A.; Abrahamsen, L.; Kim, H.J.; Kim, H.S.; Kirkerhead, C.; Kaplan, D.L. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials 2008, 29, 3415–3428. [Google Scholar] [CrossRef]
- Basnett, P. Biosynthesis of Polyhydroxyalkanoates, Their Novel Blends and Composites for Biomedical Applications. Ph.D. Thesis, University of Westminster, London, UK, 2014. [Google Scholar]
- Chen, G.Q.; Patel, M.K. Plastics derived from biological sources: Present and future: A technical and environmental review. Chem. Rev. 2012, 112, 2082–2099. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Wu, Q.; Xi, J.Z.; Yu, H.P. Microbial production of biopolyesters-polyhydroxyalkanoates. Prog. Nat. Sci. Mater. Int. 2000, 10, 847–850. [Google Scholar]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Reddy, C.S.K.; Ghai, R.; Kalia, V.C.R. Polyhydroxyalkanoates: An overview. Bioresour. Technol. 2003, 87, 137–146. [Google Scholar] [CrossRef]
- Patel, A.; Gaharwar, A.K.; Iviglia, G.; Zhang, H.; Mukundan, S.; Mihaila, S.M.; Demarchi, D.; Khademhosseini, A. Highly elastomeric poly(glycerol sebacate)-co-poly(ethylene glycol) amphiphilic block copolymers. Biomaterials 2013, 34, 3970–3983. [Google Scholar] [CrossRef]
- Yang, Z.; Galloway, J.A.; Yu, H. Protein Interactions with Poly(ethylene glycol) Self-Assembled Monolayers on Glass Substrates: Diffusion and Adsorption. Langmuir 1999, 15, 8405–8411. [Google Scholar] [CrossRef]
- Heuberger, M.; Drobek, T.; Spencer, N.D. Interaction forces and morphology of a protein-resistant poly(ethylene glycol) layer. Biophys. J. 2005, 88, 495–504. [Google Scholar] [CrossRef]
- Wei, J.; Ravn, D.B.; Gram, L.; Kingshott, P. Stainless steel modified with poly(ethylene glycol) can prevent protein adsorption but not bacterial adhesion. Colloids Surf. B Biointerfaces 2003, 32, 275–291. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, L.; Ding, M.; Tan, H.; Li, J.; Fu, Q. Preparation and rapid degradation of nontoxic biodegradable polyurethanes based on poly(lactic acid)-poly(ethylene glycol)-poly(lactic acid) andl-lysine diisocyanate. Polym. Chem. 2011, 2, 601–607. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, Y.; Sun, Y.; Fan, J.; Qin, Q.; Zhao, Z. A novel biodegradable polyurethane based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(ethylene glycol) as promising biomaterials with the improvement of mechanical properties and hemocompatibility. Polym. Chem. 2016, 7, 6120–6132. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, L.; Ding, M.; Li, J.; Tan, H.; Wang, Z.; Fu, Q. Synthesis and Characterization of pH-Sensitive Biodegradable Polyurethane for Potential Drug Delivery Applications. Macromolecules 2011, 44, 857–864. [Google Scholar] [CrossRef]
- Teo, L.S.; Chen, C.Y.; Kuo, J.F. Fourier transform infrared spectroscopy study on effects of temperature on hydrogen bonding in amine-containing polyurethanes and poly(urethane-urea)s. Macromolecules 1997, 30, 1793–1799. [Google Scholar] [CrossRef]
- Ning, N.; Yan, B.; Liu, S.; Yao, Y.; Zhang, L.; Chan, T.W.; Nishi, T.; Tian, M. Improved actuated strain of dielectric elastomer through disruption of hydrogen bonds of thermoplastic polyurethane by adding diaminonaphthalene. Smart Mater. Struct. 2015, 24, 032002. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, Y.; Xie, Y.; Qiao, K.; Sun, Y.; Yue, L. Synthesis of bio-castor oil polyurethane flexible foams and the influence of biotic component on their performance. J. Polym. Res. 2015, 22, 145. [Google Scholar] [CrossRef]
- Hong, Y.; Guan, J.; Fujimoto, K.L.; Hashizume, R.; Pelinescu, A.L.; Wagner, W.R. Tailoring the degradation kinetics of poly(ester carbonate urethane)urea thermoplastic elastomers for tissue engineering scaffolds. Biomaterials 2010, 31, 4249–4258. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Li, J.; Guo, M.; Du, R.; Xie, X.; Zhong, Y.; Fu, Q. Phase behavior and hydrogen bonding in biomembrane mimicing polyurethanes with long side chain fluorinated alkyl phosphatidylcholine polar head groups attached to hard block. Polymer 2005, 46, 7230–7239. [Google Scholar] [CrossRef]
- Yilgor, I.; Yilgor, E.; Das, S.; Wilkes, G.L. Time-dependent morphology development in segmented polyetherurea copolymers based on aromatic diisocyanates. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 471–483. [Google Scholar] [CrossRef]
- Syed Adam, S.N.F.; Osman, A.F.; Shamsudin, R. Tensile Properties, Biodegradability and Bioactivity of Thermoplastic Starch (TPS)/ Bioglass Composites for Bone Tissue Engineering. Sains Malays. 2018, 47, 1303–1310. [Google Scholar] [CrossRef]
- Al-Sanabani, J.S.; Madfa, A.A.; Al-Sanabani, F.A. Application of calcium phosphate materials in dentistry. Int. J. Biomater. 2013, 2013, 876132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Li, P.; Chen, C.B.; Wang, Z.H.; Ma, P.; Chen, G.Q. The improvement of fibroblast growth on hydrophobic biopolyesters by coating with polyhydroxyalkanoate granule binding protein PhaP fused with cell adhesion motif RGD. Biomaterials 2010, 31, 8921–8930. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, A.Z.; Zheng, A.W.; Lou, A.Z.; Chen, G.-Q. Crystallization and initial X-ray analysis of polyhydroxyalkanoate granule-associated protein from Aeromonas hydrophila. Acta Crystallogr. 2006, 62, 814–819. [Google Scholar]
- Guo, S.; Zhu, X.; Xian, J.L. Controlling cell adhesion using layer-by-layer approaches for biomedical applications. Mater. Sci. Eng. C 2017, 70, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Luo, Q.; Li, Y.; Giotto, M.; Cipollini, N.; Yang, Z.; Weiss, R.; Scola, D. Dual Crosslinked Phenylethynyl End-Capped Sulfonated Polyimides via the Ethynyl and Sulfonate Groups Promoted by PEG. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4476–4491. [Google Scholar] [CrossRef]












| Samples | Feed Ratio (mmol) | Molecular Weights | |||||
|---|---|---|---|---|---|---|---|
| LDI | PEG | PHA-diol | NCO/OH | Mw (g mol−1) | Mn (g mol−1) | Mw/Mn | |
| LPH-0 | 2 | 1 | 0 | 2 | 25,800 | 24,500 | 1.05 |
| LPH-2 | 2 | 1 | 0.2 | 1.67 | 53,600 | 42,200 | 1.27 |
| LPH-4 | 2 | 1 | 0.4 | 1.43 | 125,000 | 116,000 | 1.08 |
| LPH-6 | 2 | 1 | 0.6 | 1.25 | 176,000 | 137,000 | 1.28 |
| LPH-8 | 2 | 1 | 0.8 | 1.11 | 202,000 | 152,000 | 1.33 |
| Sample | T5% (°C) | T20% (°C) | T80% (°C) | Tm (°C) | ∆Hm (J/g) |
|---|---|---|---|---|---|
| LPH-0 | 241 | 342 | 412 | 38.3 | 55.93 |
| LPH-2 | 134 | 338 | 419 | 38.1 | 50.15 |
| LPH-4 | 275 | 351 | 419 | 36.2 | 45.32 |
| LPH-6 | 264 | 351 | 418 | 33.7 | 39.15 |
| LPH-8 | 268 | 347 | 419 | 31.4 | 46.61 |
| Sample Code | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| LPH-0 | 3.07 ± 0.4 | 1.01 ± 0.06 | 102.79 ± 6.4 |
| LPH-2 | 9.01 ± 1.1 | 5.67 ± 0.08 | 338.11 ± 16.2 |
| LPH-4 | 10.65 ± 1.8 | 7.86 ± 0.18 | 998.64 ± 32.3 |
| LPH-6 | 11.21 ± 1.6 | 8.07 ± 0.14 | 863.01 ± 19.9 |
| LPH-8 | 25.61 ± 2.7 | 9.49 ± 0.17 | 424.01 ± 28.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Xie, J.; Xiao, X.; Chen, S.; Wang, Y. Development of Nontoxic Biodegradable Polyurethanes Based on Polyhydroxyalkanoate and L-lysine Diisocyanate with Improved Mechanical Properties as New Elastomers Scaffolds. Polymers 2019, 11, 1927. https://doi.org/10.3390/polym11121927
Wang C, Xie J, Xiao X, Chen S, Wang Y. Development of Nontoxic Biodegradable Polyurethanes Based on Polyhydroxyalkanoate and L-lysine Diisocyanate with Improved Mechanical Properties as New Elastomers Scaffolds. Polymers. 2019; 11(12):1927. https://doi.org/10.3390/polym11121927
Chicago/Turabian StyleWang, Cai, Jiapeng Xie, Xuan Xiao, Shaojun Chen, and Yiping Wang. 2019. "Development of Nontoxic Biodegradable Polyurethanes Based on Polyhydroxyalkanoate and L-lysine Diisocyanate with Improved Mechanical Properties as New Elastomers Scaffolds" Polymers 11, no. 12: 1927. https://doi.org/10.3390/polym11121927
APA StyleWang, C., Xie, J., Xiao, X., Chen, S., & Wang, Y. (2019). Development of Nontoxic Biodegradable Polyurethanes Based on Polyhydroxyalkanoate and L-lysine Diisocyanate with Improved Mechanical Properties as New Elastomers Scaffolds. Polymers, 11(12), 1927. https://doi.org/10.3390/polym11121927