Fabrication of Core-Shell Magnetic Molecularly Imprinted Nanospheres towards Hypericin via Click Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Monomers and Crosslinkers
2.3. Preparation of Fe3O4 MNPs
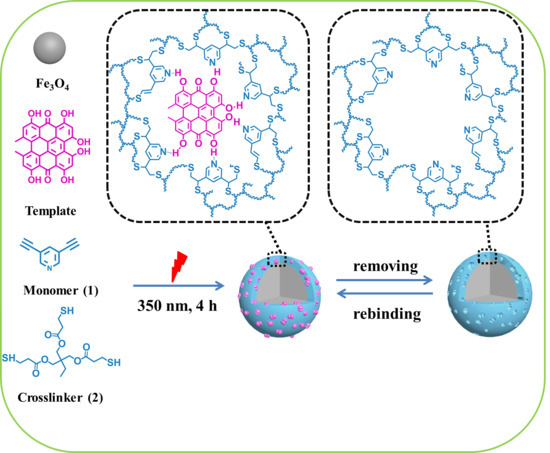
2.4. Preparation of Core-Shell Molecularly Imprinted Polymer Magnetic Nanospheres towards Hyp (Fe3O4@MIPs) via Click Reaction
2.5. Determination of Static Adsorption Capacity
2.6. Dynamic Adsorption Test
2.7. Isotherm Adsorption
2.8. Selectivity of Fe3O4@MIPs and Fe3O4@NIPs for Hyp
2.9. The Reusability of Fe3O4@MIPs
2.10. Brunauer–Emmet–Teller Analysis
2.11. HPLC Analysis
3. Results and Discussion
3.1. Preparation of Fe3O4@MIPs
3.2. Characterization of Fe3O4@MIPs
3.2.1. FTIR Analysis
3.2.2. Morphological Features
3.2.3. BET Analysis
3.3. Dynamic Adsorption Study
3.4. Affinity Analysis
3.5. Binding Selectivity of Fe3O4@MIPs to the Template Molecule Hyp
3.6. Reproducibility and Reusability of Fe3O4@MIPs
3.7. Adsorption of Fe3O4@MIPs toward Hyp from the Herb Extract
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mosbach, K.; Ramstrom, O. The emerging technique of molecular imprinting and its future impact on biotechnology. Bio-Technol. 1996, 14, 163–170. [Google Scholar] [CrossRef]
- Li, P.F.; Wang, T.; Lei, F.H.; Peng, X.Y.; Wang, H.Y.; Qin, L.T.; Jiang, J.X. Preparation and evaluation of paclitaxel-imprinted polymers with a rosin-based crosslinker as the stationary phase in high-performance liquid chromatography. J. Chromatogr. A 2017, 1502, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Walshe, M.; Howarth, J.; Kelly, M.T.; OKennedy, R.; Smyth, M.R. The preparation of a molecular imprinted polymer to 7-hydroxycoumarin and its use as a solid-phase extraction material. J. Pharmaceut. Biomed. 1997, 16, 319–325. [Google Scholar] [CrossRef]
- Sun, G.Y.; Liu, Y.F.; Ahat, H.J.; Shen, A.; Liang, X.M.; Xue, X.Y.; Luo, Y.Q.; Yang, P.; Liu, Z.S.; Aisa, H.A. “Two-dimensional” molecularly imprinted solid-phase extraction coupled with crystallization and high performance liquid chromatography for fast semi-preparative purification of tannins from pomegranate husk extract. J. Chromatogr. A 2017, 1505, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Piletsky, S.A.; Parhometz, Y.P.; Lavryk, N.V.; Panasyuk, T.L.; Elskaya, A.V. Sensors for low-weight organic-molecules based on molecular imprinting technique. Sens. Actuat. B-Chem. 1994, 19, 629–631. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, T.Y. A dual-template imprinted capsule with remarkably enhanced catalytic activity for pesticide degradation and elimination simultaneously. Chem. Commun. 2013, 49, 1073–1075. [Google Scholar] [CrossRef]
- Muldoon, M.T.; Stanker, L.H. Application of molecularly-imprinted polymers for rapid sample cleanup: Immunochemical and HPLC analysis. Arab. J. Sci. Eng. 2014, 39, 2561–2572. [Google Scholar]
- Norell, M.C.; Andersson, H.S.; Nicholls, I.A. Theophylline molecularly imprinted polymer dissociation kinetics: A novel sustained release drug dosage mechanism. J. Mol. Recognit. 1998, 11, 98–102. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Concheiro, A. Molecularly imprinted polymers for drug delivery. J. Chromatogr. B 2004, 804, 231–245. [Google Scholar] [CrossRef]
- Cunliffe, D.; Kirby, A.; Alexander, C. Molecularly imprinted drug delivery systems. Adv. Drug. Deliver. Rev. 2005, 57, 1836–1853. [Google Scholar] [CrossRef]
- Pardeshi, S.; Singh, S.K. Precipitation polymerization: A versatile tool for preparing molecularly imprinted polymer beads for chromatography applications. RSC Adv. 2016, 6, 23525–23536. [Google Scholar] [CrossRef]
- Cederfur, J.; Pei, Y.X.; Meng, Z.H.; Kempe, M. Synthesis and screening of a molecularly imprinted polymer library targeted for penicillin g. J. Comb. Chem. 2003, 5, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Niu, D.C.; Li, Y.S.; Shi, J.L. Magnetic, core-shell structured and surface molecularly imprinted polymers for the rapid and selective recognition of salicylic acid from aqueous solutions. Appl. Surf. Sci. 2018, 435, 178–186. [Google Scholar] [CrossRef]
- Gu, X.H.; Xu, R.; Yuan, G.L.; Lu, H.; Gu, B.R.; Xie, H.P. Preparation of chlorogenic acid surface-imprinted magnetic nanoparticles and their usage in separation of traditional chinese medicine. Anal. Chim. Acta 2010, 675, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, A.A.; Ersoz, A.; Hur, D.; Yilmaz, F.; Gultekin, A.; Denizli, A.; Say, R. Semi-synthetic biotin imprinting onto avidin crosslinked gold-silver nanoparticles. J. Nanopart. Res. 2012, 14, 945. [Google Scholar] [CrossRef]
- Ma, J.; Yuan, L.H.; Ding, M.J.; Wang, S.; Ren, F.; Zhang, J.; Du, S.H.; Li, F.; Zhou, X.M. The study of core-shell molecularly imprinted polymers of 17 beta-estradiol on the surface of silica nanoparticles. Biosens. Bioelectron. 2011, 26, 2791–2795. [Google Scholar] [CrossRef] [PubMed]
- Machynakova, A.; Hrobonova, K. Preparation and application of magnetic molecularly imprinted polymers for the selective extraction of coumarins from food and plant samples. Anal. Methods-UK 2017, 9, 2168–2176. [Google Scholar] [CrossRef]
- Harrer, G.; Sommer, H. Treatment of mild/moderate depressions with Hypericum. Phytomed. Int. J. Phytother. Phytopharm. 1994, 1, 3–8. [Google Scholar] [CrossRef]
- Asgarpanah, J. Phytochemistry, pharmacology and medicinal properties of Hypericum perforatum L. Afr. J. Pharm. Pharmaco. 2012, 6, 1387–1394. [Google Scholar] [CrossRef]
- Yip, L.; Hudson, J.B.; GruszeckaKowalik, E.; Zalkow, L.H.; Towers, G.H.N. Antiviral activity of a derivative of the photosensitive compound hypericin. Phytomedicine 1996, 3, 185–190. [Google Scholar] [CrossRef]
- Stojanovic, G.; Dordevic, A.; Smelcerovic, A. Do other hypericum species have medical potential as St. John’s wort (Hypericum perforatum)? Curr. Med. Chem. 2013, 20, 2273–2295. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Z.; Qin, C.L.; Li, D.M.; Hou, Y.Z.; Li, S.B.; Sun, J.J. Molecularly imprinted polymer for specific extraction of hypericin from Hypericum perforatum L. Herbal extract. J. Pharmaceut. Biomed. 2014, 98, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.X.; Fan, F.F.; Zhang, Y.; Pei, Z.C.; Wang, W.J.; Pei, Y.X. A facile approach for fabrication of core-shell magnetic molecularly imprinted nanospheres towards hypericin. Polymers 2017, 9, 135. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Xu, C.G.; Shen, X.T.; Ye, L. Molecularly imprinted magnetic materials prepared from modular and clickable nanoparticles. J. Mater. Chem. 2012, 22, 7427–7433. [Google Scholar] [CrossRef]
- Stephenson-Brown, A.; Acton, A.L.; Preece, J.A.; Fossey, J.S.; Mendes, P.M. Selective glycoprotein detection through covalent templating and allosteric click-imprinting. Chem. Sci. 2015, 6, 5114–5119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.F.; Deng, P.H.; Tang, S.P.; Kuang, D.Z. Preparation of 2D molecularly imprinted materials based on mesoporous silicas via click reaction. J. Mater. Chem. B 2014, 2, 8418–8426. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, J.P.; He, J.L.; Deng, Q.L.; Wang, S. One-step post-imprint modification achieve dual-function of glycoprotein fluorescent sensor by “click chemistry”. Biosens. Bioelectron. 2017, 91, 756–761. [Google Scholar] [CrossRef]
- Hou, Y.; Cao, S.P.; Li, X.M.; Wang, B.B.; Pei, Y.X.; Wang, L.; Pei, Z.C. One-step synthesis of dual clickable nanospheres via ultrasonic-assisted click polymerization for biological applications. Appl. Mater. Inter. 2014, 6, 16909–16917. [Google Scholar] [CrossRef]
- Hou, Y.; Cao, S.P.; Wang, L.; Pei, Y.X.; Zhang, G.Y.; Zhang, S.W.; Pei, Z.C. Morphology-controlled dual clickable nanoparticles via ultrasonic-assisted click polymerization. Polym. Chem.-UK 2015, 6, 223–227. [Google Scholar] [CrossRef]
- Pei, Y.X.; Fan, F.F.; Wang, X.X.; Feng, W.W.; Hou, Y.; Pei, Z.C. Fabrication of hypericin imprinted polymer nanospheres via thiol-yne click reaction. Polymers 2017, 9, 469. [Google Scholar] [CrossRef]
- Ansell, R.J. Characterization of the Binding Properties of Molecularly Imprinted Polymers. Adv. Biochem. Eng. Biotechnol. 2015, 150, 51–93. [Google Scholar] [PubMed] [Green Version]
- Zhang, Y.; Shang, K.; Wu, X.W.; Song, S.Y.; Li, Z.B.; Pei, Z.C.; Pei, Y.X. Highly efficient green synthesis and photodynamic therapeutic study of hypericin and its derivatives. RSC Adv. 2018, 8, 21786–21792. [Google Scholar] [CrossRef] [Green Version]
- Jin, F.; Zheng, M.L.; Zhang, M.L.; Zhao, Z.S.; Duan, X.M. A facile layer-by-layer assembly method for the fabrication of fluorescent polymer/quantum dot nanocomposite thin films. RSC Adv. 2014, 4, 33206–33214. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.L.; Peng, Q.; Wang, X.; Chen, J.P.; Li, Y.D. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 2010, 44, 2782–2785. [Google Scholar] [CrossRef] [PubMed]
- Senhadjikebiche, O.; Belaid, T.; Benamor, M. Preparation and characterization of molecularly imprinted polymer as spe sorbent for melamine isolation. Polymers 2013, 5, 1215–1228. [Google Scholar]
- Kupai, J.; Rojik, E.; Huszthy, P.; Szekely, G. Role of chirality and macroring in imprinted polymers with nantiodiscriminative power. Appl. Mater. Interfaces 2015, 7, 9516–9952. [Google Scholar] [CrossRef]











| Nanospheres | Paticle Size (nm) | Polydispersity Index | ζ Potential (mV) |
|---|---|---|---|
| MNPs | 309 ± 61 | 0.271 | 1.35 ± 0.17 |
| Fe3O4@MIPs | 344 ± 55 | 0.386 | 3.49 ± 0.72 |
| Fe3O4@NIPs | 320 ± 75 | 0.315 | 2.06 ± 0.86 |
| Fe3O4@MIPs after extracting process | 323 ± 80 | 0.646 | −3.58 ± 0.58 |
| Fe3O4@NIPs after extracting process | 314 ± 46 | 0.378 | 0.20 ± 0.69 |
| Nanospheres | Average Pore Diameter (nm) | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) |
|---|---|---|---|
| Fe3O4@MIPs | 19.18 ± 0.26 | 8.31 ± 0.25 | 0.04 ± 0.03 |
| Fe3O4@NIPs | 7.84 ± 0.12 | 2.81 ± 0.01 | 0.004 ± 0.01 |
| Factor | Hyp | Protohyp | Emodin |
|---|---|---|---|
| SF | - | 4.16 | 7.88 |
| IF | 9.93 | 3.11 | 2.41 |
| Sample | Peak Area (Hyp) | Peak Area (Protohyp) | Adsorption of Hyp (%) | Adsorption of Protohyp (%) |
|---|---|---|---|---|
| Initial | 141,375 | 80,769 | - | - |
| Fe3O4@NPs | 115,094 | 64,398 | 18.6 | 20.3 |
| Fe3O4@MIPs | 75,618 | 63,397 | 46.5 | 21.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Pei, Y.; Hou, Y.; Pei, Z. Fabrication of Core-Shell Magnetic Molecularly Imprinted Nanospheres towards Hypericin via Click Polymerization. Polymers 2019, 11, 313. https://doi.org/10.3390/polym11020313
Wang X, Pei Y, Hou Y, Pei Z. Fabrication of Core-Shell Magnetic Molecularly Imprinted Nanospheres towards Hypericin via Click Polymerization. Polymers. 2019; 11(2):313. https://doi.org/10.3390/polym11020313
Chicago/Turabian StyleWang, Xinxin, Yuxin Pei, Yong Hou, and Zhichao Pei. 2019. "Fabrication of Core-Shell Magnetic Molecularly Imprinted Nanospheres towards Hypericin via Click Polymerization" Polymers 11, no. 2: 313. https://doi.org/10.3390/polym11020313
APA StyleWang, X., Pei, Y., Hou, Y., & Pei, Z. (2019). Fabrication of Core-Shell Magnetic Molecularly Imprinted Nanospheres towards Hypericin via Click Polymerization. Polymers, 11(2), 313. https://doi.org/10.3390/polym11020313