New Alginate/PNIPAAm Matrices for Drug Delivery
Abstract
:1. Introduction
2. Experimental
2.1. Materials
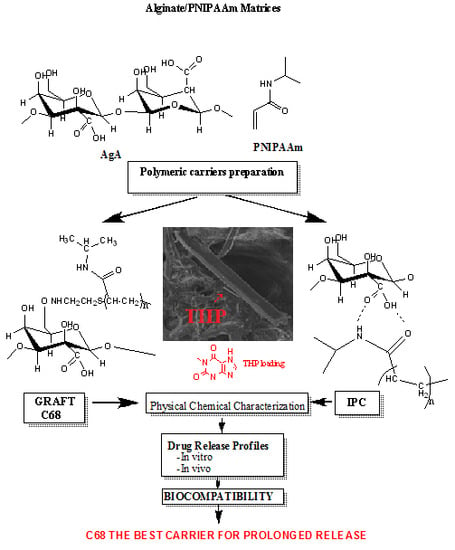
- An interpolymeric complex (IPC) of AgA/PNIPAAm as previously described by solutions mixing using “iso pH” method [18,19]. The diluted aqueous both polymer solutions of equal concentration (0.5 wt %) and at the same pH (e.g., of 5.5) were mixed in various ratios (15/85; 30/70, 41/59 and 72/28 wt % AgA/wt % PNIPAAm). These ratios were previously established as the most stable associations by hydrogen bonding. The freeze-dried IPCs of various compositions were separated from solutions. The optimal composition AgA/PNIPAam 72/28 (w/w %) (IPC 72/28) was selected for this study.
- Graft AgA-g-PNIPAAm copolymers were synthesized and characterized as reported in previous studies [21,22,24] by using a method similar with that applied for other types of copolymers [25,26,27,28]. Grafting of PNIPAM-NH2 chains onto sodium alginate, NaAgA, was realized by using 1-3-(3-dimethylaminopropyl)–3-ethyl-carbodiimide hydrochloride (EDC) (Sigma Aldrich Chemie GmbH, Export Department, Taufkirchen, Germany) 98% as a condensing agent in the presence of 1-hydroxibenzotriazole hydrate (HOBt) (FlukaHoneywell International Inc., by VWR International GmbH, Wien, Austria)) as a coupling agent. The grafting reaction occurred through an amide group formed from the carboxylate groups of sodium alginate (NaAgA) and the amine group of the amine-terminated PNIPAAm. The graft copolymers obtained were purified by several successive precipitations in acetone and finally freeze-dried [21,22,24,28]. The composition of the graft copolymers obtained was assessed by 1H nuclear magnetic resonance (NMR) spectroscopy (BRUKER AVANCE DRX 400 MHz apparatus, Billerica, MA, USA), using D2O as solvent. The content in PNIPAAm found varied from 30% to 68% and average viscosity molecular weight (Mv) of the PNIPAAm side chains were about 19–35 kDa.
- Suspensions in water and carboxymethyl cellulose (5 wt %) for the in vivo drug release.
- Lyophilized form for the physical chemical characterization.
2.2. Preparation Methods
Preparation of Theophylline-Loaded Samples
2.3. Investigation Methods
2.3.1. Near Infrared Spectroscopy–Chemical Imaging (NIR–CI)
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Ethics Statement for Experiments with Animals
2.3.5. Toxicity and Biocompatibility Studies
2.3.6. Statistical Analysis
2.3.7. In Vitro Theophylline Release
2.3.8. In Vivo Theophylline Release
3. Results and Discussions
3.1. Naer Infrared (NIR) Results
3.2. Scanning Electron Microscope (SEM) Images
3.3. TGA Results
3.4. Toxicity and Biocompatibility Evaluation
3.4.1. Toxicity Tests
3.4.2. Biocompatibility Studies
3.5. Drug Release Results
3.5.1. In Vitro Release of Theophylline
3.5.2. In Vivo Theophylline Release
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haque, S.; Whittaker, M.R.; McIntosh, M.P.; Pouton, C.W.; Kaminskas, L.M. Disposition and safety of inhaled biodegradable nanomedicines: Opportunities and challenges. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1703–1724. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.I.R.; Garcia-Contreras, L. Inhalation drug delivery devices: Technology update. Med. Devices Evid. Res. 2015, 8, 131–139. [Google Scholar]
- Carrier, J.A.; Shaw, R.A.; Porter, R.S.; Allison, E.J.; Kessler, E.R.; Woody, D.G.; Harker, C.C.; Jones, J.G. Comparison of intravenous and oral routes of theophylline loading in acute asthma. Ann. Emerg. Med. 1985, 14, 1145–1151. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Li, J.; Xu, W.; Li, D.; Liu, T.; Zhang, Y.S.; Ding, J.; Chen, X. Locally Deployable Nanofiber Patch for Sequential Drug Delivery in Treatment of Primary and Advanced Orthotopic Hepatomas. ACS Nano 2018, 12, 6685–6699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, W.; Ning, C.; Li, M.; Zhao, G.; Jiang, W.; Ding, J.; Chen, X. Long-acting hydrogel/microsphere composite sequentially releases dexmedetomidine and bupivacaine for prolonged synergistic analgesia. Biomaterials 2018, 181, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ning, C.; Xu, W.; Hu, H.; Li, M.; Zhao, G.; Ding, J.; Chen, X. Precision-guided long-acting analgesia by Gel-immobilized bupivacaine-loaded microsphere. Theranostics 2018, 8, 3331–3347. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, T.; Xu, W.; Ding, J.; Yin, F.; Xu, J.; Sun, W.; Wang, H.; Sun, M.; Cai, Z.; Hua, Y. Sarcoma-Targeting Peptide-Decorated Polypeptide Nanogel Intracellularly Delivers Shikonin for Upregulated Osteosarcoma Necroptosis and Diminished Pulmonary Metastasis. Theranostics 2018, 8, 1361–1375. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, J.; Xu, W.; Xiao, G.; Chen, X. Tumor microenvironment-labile polymer–doxorubicin conjugate thermogel combined with docetaxel for in situ synergistic chemotherapy of hepatoma. Acta Biomater. 2018, 77, 63–73. [Google Scholar] [CrossRef]
- Liang, Z.; Ni, R.; Zhou, J.; Mao, S. Recent advances in controlled pulmonary drug delivery. Drug Discov. Today 2015, 20, 380–389. [Google Scholar] [CrossRef]
- Tonnesen, H.H.; Karlsen, J. Alginate in Drug Delivery Systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosnik, A. Alginate Particles as Platform for Drug Delivery by the Oral Route: State-of-the-Art. ISRN Pharm. 2014. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Chang, R.-K. Review of current issues in pharmaceutical excipients. Pharm. Technol. 2006, 31, 56–66. [Google Scholar]
- Sabra, W.; Deckwer, W.-D. Alginate-A polysaccharide of industrial interest and diverse biological functions. In Polysaccharides: Structural Diversity and Functional Versatility; Dumitriu, S., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2005; Chapter 21; pp. 515–534. ISBN 0-8247-5480-8. [Google Scholar]
- Ogilvie, R.I. Clinical pharmacokinetics of theophylline. Clin. Pharmacokinet. 1978, 3, 267–293. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, R.P.; Mitchell, G.R.; Vasile, C. Rheological and thermal behaviour of poly(N-isopropyl acryl amide)/alginate smart polymeric networks. Polym. Int. 2011, 60, 1398–1407. [Google Scholar] [CrossRef]
- Dumitriu, R.P.; Mitchell, G.R.; Vasile, C. Multi-responsive hydrogels based on N-isopropylacrylamide and sodium alginate. Polym. Int. 2011, 60, 222–233. [Google Scholar] [CrossRef]
- Cheaburu, C.N.; Vasile, C. Responsive Freeze-Drying Interpolymeric Associations of Alginic Acid and Poly(N-isopropyl acrylamide) II. The Dependence of the Transition Temperature on pH and composition. Cell. Chem. Technol. 2008, 42, 207–212. [Google Scholar]
- Duncianu, C.N.; Vasile, C. Interpolymeric Associations Between Alginic Acid and Poly(N-Isopropylacrylamide), Poly(Ethylene Glycol) and Polyacrylamide. Polym. Res. J. 2008, 2, 181–200. [Google Scholar]
- Vasile, C.; Dumitriu, R.P.; Cheaburu, C.N.; Oprea, A.M. Architecture and composition influence on the properties of some smart polymeric materials designed as matrices in drug delivery systems. A comparative study. Appl. Surf. Sci. 2009, 256, 65–71. [Google Scholar] [CrossRef]
- Vasile, C.; Nita, L.E. Novel multi-stimuli responsive sodium alginate-grafted-poly(N-isopropyl acryl amide) copolymers: II. Dilute solution properties. Carbohydr. Polym. 2011, 86, 77–84. [Google Scholar] [CrossRef]
- Cheaburu, C.N.; Ciocoiu, O.N.; Staikos, G.; Vasile, C. Thermoresponsive sodium alginate-g-poly(N-isopropyl acryl amide) copolymers. III. Solution properties. J. App. Polym. Sci. 2013, 127, 3340–3348. [Google Scholar] [CrossRef]
- Dumitriu, R.P.; Oprea, A.M.; Cheaburu, C.N.; Nistor, M.T.; Novac, O.; Ghiciuc, C.M.; Profire, L.; Vasile, C. Biocompatible and Biodegradable Alginate/Poly(N-isopropyl acryl amide) Hydrogels for Sustained Theophylline Release. J. Appl. Polym. Sci. 2014, 131, 40733–40748. [Google Scholar] [CrossRef]
- Ciocoiu, O.N.; Staikos, G. Sodium Algınate-Graft-Poly(N-Isopropylacrylamide) Copolymers As Thickening Agents. 2013, 5, pp. 23–25. Available online: http://9pesxm.chemeng.ntua.gr/_view_paper/117 (accessed on 19 February 2019).
- Bokias, G.; Mylonas, Y.; Staikos, G.; Bumbu, G.G.; Vasile, C. Synthesis and Aqueous Solution Properties of Novel Thermoresponsive Graft Copolymers Based on a Carboxymethylcellulose Backbone. Macromolecules 2001, 34, 4958–4964. [Google Scholar] [CrossRef]
- Vasile, C.; Bumbu, G.G.; Mylonas, I.; Bokias, G.; Staikos, G. Thermoresponsive behaviour in aqueous solution of poly(maleic acid-alt-vinyl acetate) grafted with poly(N-isopropyl acryl amide). Polym. Int. 2004, 53, 1176–1179. [Google Scholar] [CrossRef]
- Ciocoiu, O.N.; Vasile, C.; Staikos, G. Thermoresponsive behavior of sodium alginate grafted with poly(N-isopropyl acryl amide) in aqueous media. Carbohydr. Polym. 2018, 184, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Cheaburu-Yilmaz, C.N.; Vasile, C.; Ciocoiu, O.-N.; Staikos, G. Sodium alginate grafted with poly(N-isopropyl acryl amide). In Temperature-Responsive Polymers: Chemistry, Properties and Applications; Khutoryanskiy, V., Georgiou, T., Eds.; Wiley & Sons: San Francisco, CA, USA, 2018; Chapter 6; pp. 121–145. ISBN 978-1-119-15778-6. [Google Scholar]
- AVMA Guidelines for the Euthanasia of Animals. Available online: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf (accessed on 17 February 2019).
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef] [Green Version]
- Popa, N.; Novac, O.; Profire, L.; Lupusoru, E.; Popa, M.I. Hydrogels based on chitosan–xanthan for controlled release of theophylline. J. Mater. Sci. Mater. Med. 2010, 21, 1241–1248. [Google Scholar] [CrossRef]
- Cheaburu-Yilmaz, C.N.; Dumitriu, R.P.; Nistor, M.T.; Lupusoru, C.; Popa, M.I.; Profire, L.; Silvestre, C.; Vasile, C. Biocompatible and Biodegradable Chitosan/Clay Nanocomposites as New Carriers for Theophylline Controlled Release. Br. J. Pharm. Res. 2015, 6, 228–254. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Lustig, S.R.; Peppas, N.A. Solute and penetrant diffusion in swellable polymers. I.Mathematical modeling. J. Polym. Sci. Part B Polym. Phys. 1986, 24, 395–408. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release. II Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Ram, F.S.F.; Jardin, J.R.; Atallah, A.; Castro, A.A.; Mazzini, R.; Goldstein, R. Efficacy of theophylline in people with stable chronic obstructive pulmonary disease: A systematic review and meta-analysis. Respir. Med. 2005, 99, 135–144. [Google Scholar] [CrossRef]
- ASTM International. ASTM F 756-00Standard Practice. Assessment of Hemolytic Properties of Materials, 2017 ed.; March 1, 2017 ICS Code (Laboratory medicine):11.100; ASTM International: West Conshohocken, PA, USA, 2017; p. 6. [Google Scholar]
- OECD/OCDE Guidelines for the Testing of Chemicals, Acute Oral Toxicity—Up-and-Down-Procedure (UDP); Château de la Muette: Paris, France, 2001. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd_gl425-508.pdf (accessed on 17 February 2019).
- Schneck, K.; Washington, M.; Holder, D.; Lodge, K.; Motzel, S. Hematologic and serum biochemical reference values in nontransgenic FVB mice. Comp. Med. 2000, 50, 32–35. [Google Scholar]
- Kawai, M.; Kato, M. Theophylline for the treatment of bronchial asthma: Present status. Methods Find Exp. Clin. Pharm. 2000, 22, 309–320. [Google Scholar] [CrossRef]
- Nelson, L.S.; Ford, M.D. Acute poisoning. In Goldman-Cecil Medicine, 25th ed.; Goldman, L., Schafer, A.I., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2016; Chapter 110. [Google Scholar]
- Pincus, M.R.; Abraham, N.Z. Toxicology and Therapeutic Drug Monitoring. In Henry’s Clinical Diagnosis and Management by Laboratory Methods, 23rd ed.; McPherson, R.A., Pincus, M.R., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2017; Chapter 23; 1700p, ISBN 978-0-323-41315-2. [Google Scholar]
- Diasio, R.B. Principles of drug therapy. In Goldman-Cecil Medicine, 24th ed.; Goldman, L., Schafer, A.I., Eds.; Elsevier Inc.: Philadelphia, PA, USA, 2011; Chapter 29; pp. 124–132. [Google Scholar]







| Sample | Polymeric Matrix (wt %) | Unknown Compound (wt %) | Drug Loading (%) Raported to Initial THP Amount |
|---|---|---|---|
| IPC 72/28-THP | 98.32 ± 4.91 | 1.68 ± 0.08 | 42 ± 2.10 |
| C25-THP | 98.20 ± 4.90 | 1.80 ± 0.09 | 45 ± 2.25 |
| C43-THP | 98.47 ± 4.92 | 1.53 ± 0.07 | 39 ± 1.95 |
| C68-THP | 98.20 ± 4.90 | 1.80 ± 0.09 | 45 ± 2.25 |
| Theophylline | IPC 72/28 | AgA-g-PNIPAAm | Assignment | ||||
|---|---|---|---|---|---|---|---|
| C43 | C68 | ||||||
| Without THP | With THP | Without THP | With THP | Without THP | With THP | ||
| 1170 | 1163 | 1160 | 1152 | 1160 | 1152 | C–H second overtone | |
| 1367 | 1372 | 1361 | 1367 | 1357 | First C–H overtone and combinations | ||
| 1433 | 1437 sh | 1441 | N–H stretch 1st overtone | ||||
| 1631 sh | 1631 sh | 1631 sh | 1623s | 1635 | N–H stretch 1st overtone | ||
| 1676 | 1665 | 1673 | 1669 | 1665 | 1665 | 1665 | C–H stretch 1st overtone |
| 1710 | 1714 | 1714s | 1718 | 1707 | 1722 | 1711 | C–H stretch 1st overtone |
| 1819 1882 | 1817 sh 1878 | C–O and O–H combinations | |||||
| 1874 | 1874 | 1870 | 1870 | 1865 | |||
| 2004 | 2015 | 2015 | 2007s | 2007 | 2011 | 2015 | O–H bend second overtone |
| 2095 | 2090 | 2095 | 2090 | 2095 | 2090 | 2098 | O–H and N–H combinations |
| 2137 | 2140 | 2144 | 2136 | 2140 | 2140 | 2144 | N–H combinations |
| 2254 | 2258 | 2254 | 2209; 2258 | 2197; 2258 | 2197; 2254 | 2197; 2254 | O–H and C–H combinations |
| 2321 2376 | 2326 2376 | 2326 2376 | 2322; 2376 | 2326; 2376 | 2322; 2374 | 2330; 2372 | C–H stretch/CH2 deformation |
| 2429 2475 | 2436 2476 | 2436 2474 | 2433; 2478 | 2430 | 2430; 2478 | 2429; 2478 | C–H and C–C combinations C–N–C stretch overtone |
| Sample | Maximum Temperature (°C)—from DTGA | |||||
|---|---|---|---|---|---|---|
| Step 1 | Step 2 | Step 3 | Step 4 | Step 5 | Step 6 | |
| IPC 72/28 | 68 | 265 | 295 sh | 312 | 402 sh | 532 |
| C25 | 37; 60 | 253 | 282 | 311; 327 348; 381 | 442 | 517 |
| C43 | 42; 69 | 255 | 306; | 350; 385 sh | 452 | 500 |
| C68 | 42; 69 | 255 | 305; | 385 sh | 524 | |
| Alginate | 245; 269 | |||||
| Theophyline | 298 sh; | 337 | ||||
| PNIPAAm | 436 | |||||
| Hematological Parameter | Control Mice Group | Tested Mice Groups i.p. Injected with Suspension of: | |
|---|---|---|---|
| IPC 72/28-THP | C68-THP | ||
| White blood cells (×109/L) | 5.64 ± 0,13 | 5.6 ± 0,1 | 5.65 ± 0.11 |
| Polymorphonuclear cells (PMN) (×109/L) | 1.51 ± 0.06 | 1.51 ± 0.06 | 1.54 ± 0.04 |
| Lymphocytes (×109/L)) | 3.68 ± 0.11 | 3.64 ± 0.09 | 3.65 ± 0.1 |
| Monocytes (×109/L) | 0.35 ± 0.05 | 0.34 ± 0.05 | 0.36 ± 0.04 |
| Eosinophils (×109/L) | 0.04 ± 0.02 | 0.05 ± 0.01 | 0.04 ± 0.02 |
| Basophils (×109/L) | 0.05 ± 0.03 | 0.06 ± 0.01 | 0.05 ± 0.03 |
| Polymorphonuclear cells (PMN) (%) | 26.8 ± 0.97 | 26.94 ± 1.02 | 27.25 ± 0.5 |
| Lymphocyte (%) | 65.3 ± 1.05 | 65.34 ± 1.27 | 64.8 ± 0.52 |
| Monocytes (%) | 6.23 ± 0.71 | 6.14 ± 0.77 | 6.34 ± 0.56 |
| Eosinophils (%) | 0.78 ± 0.36 | 0.9 ± 0.19 | 0.74 ± 0.45 |
| Basophils (%) | 0.93 ± 0.47 | 1.08 ± 0.26 | 0.86 ± 0.54 |
| Red blood cells (×109/L) | 9.39 ± 0.06 | 9.4 ± 0.07 | 9.41 ± 0.09 |
| Hemoglobin level (g/dL) | 11.5 ± 0.05 | 11.45 ± 0.05 | 11.48 ± 0.06 |
| Hematocrit level (%) | 41.0 ± 0.04 | 41.08 ± 0.19 | 41.1 ± 0.2 |
| NBT test (%) | 13.8 ± 0.75 | 13.71 ± 0.76 | 13.83 ± 0.75 |
| Platelets (×109/L) | 253 ± 38.8 | 252.9 ± 16.2 | 252.99 ± 8.01 |
| Immune System Parameters | |||
| Serum opsonic capacity (S. aureus ×1000/mL) | 771.7 ± 58.4 | 774. 3 ± 53.8 | 773.3 ± 59.55 |
| Phagocytic capacity of peritoneal macrophages (S. aureus ×1000/mL) | 716.7 ± 51.6 | 728.5 ± 59.8 | 735 ± 62.85 |
| Bactericidal capacity of peritoneal macrophages (S. aureus ×1000/mL) | 696.7 ± 8.2 | 697.14 ± 9.51 | 698.33 ± 9.83 |
| Splenic T lymphocytes (%) | 12.5 ± 0.55 | 12.57 ± 0.53 | 12.67 ± 0.52 |
| TGP (UI/I) | 23.17 ± 1.17 | 24 ± 1.63 | 23.17 ± 1.17 |
| TGO (UI/I) | 73.33 ± 1.75 | 73.14 ± 1.21 | 72.33 ± 0.92 |
| LDH (UI/I) | 497.5 ± 3.33 | 498.71 ± 1.8 | 499.33 ± 3.88 |
| Sample | Maximum Amount Released (%) | teq. (min) | t1/2r (min) | nr | Rnr | kr·103 (min−1) | Rkr |
|---|---|---|---|---|---|---|---|
| IPC 72/28 | 52.40 ± 2.62 | 77.6 ± 3.88 | 25.4 ± 1.27 | 1.41 ± 0.07 | 0.98 | 10.0 ± 0.50 | 1.00 |
| C25 | 56.10 ± 2.80 | 302.2 ± 15.11 | 73.5 ± 3.67 | 0.72 ± 0.03 | 0.96 | 12.4 ± 0.62 | 0.97 |
| C43 | 56.30 ± 2.81 | 271.4 ± 13.57 | 82.9 ± 4.14 | 1.01 ± 0.05 | 0.96 | 7.6 ± 0.38 | 0.97 |
| C68 | 52.40 ± 2.62 | 235.4 ± 11.74 | 41.4 ± 2.07 | 1.80 ± 0.09 | 0.93 | 1.7 ± 0.08 | 0.97 |
| Parameter | THP | IPC 72/28 | C68 |
|---|---|---|---|
| tmax (h) | 1.03 ± 0.05 | 2.90 ± 0.14 | 3.90 ± 0.19 |
| t½ (h) | 2.50 ± 0.12 | 7.00 ± 0.35 | 12.0 ± 0.60 |
| Cmax (μg/mL) | 7.10 ± 0.35 | 3.11 ± 0.15 | 13.94 ± 0.69 |
| AUC0–72 (μg h/mL) | 38.52 ± 1.92 | 22.94 ± 1.14 | 67.43 ± 3.30 |
| Relative bioavailability (%) | - | 59.55 ± 2.97 | 175.00 ± 8.75 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheaburu-Yilmaz, C.N.; Lupuşoru, C.E.; Vasile, C. New Alginate/PNIPAAm Matrices for Drug Delivery. Polymers 2019, 11, 366. https://doi.org/10.3390/polym11020366
Cheaburu-Yilmaz CN, Lupuşoru CE, Vasile C. New Alginate/PNIPAAm Matrices for Drug Delivery. Polymers. 2019; 11(2):366. https://doi.org/10.3390/polym11020366
Chicago/Turabian StyleCheaburu-Yilmaz, Catalina N., Catalina Elena Lupuşoru, and Cornelia Vasile. 2019. "New Alginate/PNIPAAm Matrices for Drug Delivery" Polymers 11, no. 2: 366. https://doi.org/10.3390/polym11020366
APA StyleCheaburu-Yilmaz, C. N., Lupuşoru, C. E., & Vasile, C. (2019). New Alginate/PNIPAAm Matrices for Drug Delivery. Polymers, 11(2), 366. https://doi.org/10.3390/polym11020366