Multifunctional Silicone Rubber Nanocomposites by Controlling the Structure and Morphology of Graphene Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Thermally Reduced Graphene Oxide (TRGO)
2.3. Preparation of TRGO Filled Silicone Rubber Composites
2.4. Characterization of Graphene Materials and Their Silicone Composites
3. Results and Discussion
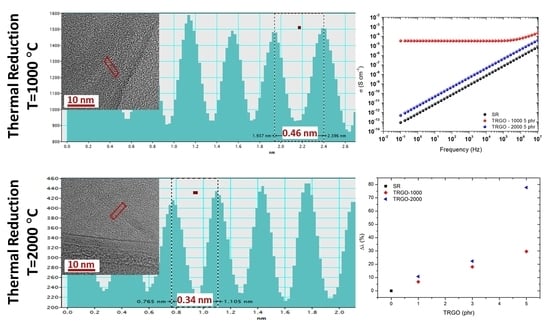
3.1. Characterization of Graphene Materials
3.2. Morphology of TRGO Filled Silicone Rubber Composites
3.3. Mechanical Properties of TRGO Filled Silicone Rubber Composites
3.4. Electrical Properties of TRGO Filled Silicone Rubber Composites
3.5. Thermal Conductive of TRGO Filled Silicone Rubber Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shit, S.C.; Shah, P. A Review on Silicone Rubber. Natl. Acad. Sci. Lett. USA 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Stieghorst, J.; Majaura, D.; Wevering, H.; Doll, T. Toward 3D Printing of Medical Implants: Reduced Lateral Droplet Spreading of Silicone Rubber under Intense IR Curing. ACS Appl. Mater. Interfaces 2016, 8, 8239–8246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, Y.; Zhang, D.; Wang, W.; Xing, Y.; Lin, J.; Hong, H.; Li, C. Graphene Nanosheet/Silicone Composite with Enhanced Thermal Conductivity and its Application in Heat Dissipation of High-Power Light-emitting Diodes. Curr. Appl. Phys. 2016, 16, 1695–1702. [Google Scholar] [CrossRef]
- Chen, C.Y.; Pu, N.W.; Liu, Y.M.; Huang, S.Y.; Wu, C.H.; Ger, M.D.; Gong, Y.J.; Chou, Y.C. Remarkable Microwave Absorption Performance of Graphene at a Very Low Loading Ratio. Compos. Part B 2017, 114, 395–403. [Google Scholar] [CrossRef]
- He, Z.; Chen, Y.; Jian Yang, J.; Tang, C.; Lv, J.; Liu, Y.; Mei, J.; Lau, W.M.; Hui, D. Fabrication of Polydimethylsiloxane Films with Special Surface Wettability by 3D Printing. Compos. Part B 2017, 129, 58–65. [Google Scholar] [CrossRef]
- Yang, H.; Yao, X.; Zheng, Z.; Gong, L.; Yuan, L.; Yuan, Y.; Liu, Y. Highly Sensitive and Stretchable Graphene-Silicone Rubber Composites for Strain Sensing. Compos. Sci. Technol. 2018, 167, 371–378. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401–187404. [Google Scholar] [CrossRef]
- Gomez-Navarro, C.; Meyer, J.C.; Sundaram, R.S.; Chuvilin, A.; Kurasch, S.; Burghard, M.; Kern, K.; Kaiser, U. Atomic Structure of Reduced Graphene Oxide. Nano Lett. 2010, 10, 1144–1148. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, And Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Song, Y.; Yu, J.; Yu, L.; Alam, F.E.; Dai, W.; Jiang, N. Enhancing the Thermal, Electrical, and Mechanical Properties of Silicone Rubber by Addition of Graphene Nanoplatelets. Mater. Des. 2015, 88, 950–957. [Google Scholar] [CrossRef]
- Shi, G.; Zhao, Z.; Pai, J.H.; Lee, I.; Zhang, L.; Stevenson, C.; Ishara, K.; Zhang, R.; Zhu, H.; Ma, J. Highly Sensitive, Wearable, Durable Strain Sensors and Stretchable Conductors Using Graphene/Silicon Rubber Composites. Adv. Funct. Mater. 2016, 26, 7614–7625. [Google Scholar] [CrossRef]
- Zhao, X.W.; Zang, C.G.; Wen, Y.G.; Jiao, Q.J. Thermal and Mechanical Properties of Liquid Silicone Rubber Composites Filled with Functionalized Graphene Oxide. J. Appl. Polym. Sci. 2015, 42582. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, F.; Dai, J.; Huang, Z. Effect of Functionalization of Graphene Nanoplatelets on the Mechanical and Thermal Properties of Silicone Rubber Composites. Materials 2016, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, B. Improvement of Thermal Conductivities for Silicone Nanocomposite Via Incorporating Poly(γ-methacryloxypropyltrimethoxy silane) Grafted Graphene Fillers. Chem. Phys. Lett. 2018, 693, 121–126. [Google Scholar] [CrossRef]
- Song, J.; Chen, C.; Zhang, Y. High Thermal Conductivity and Stretchability of Layer-by-Layer Assembled Silicone Rubber/Graphene Nanosheets Multilayered Films. Compos. Part A 2018, 105, 1–8. [Google Scholar] [CrossRef]
- Qiu, X.; Cai, H.; Fang, X.; Zheng, J. The Improved Thermal Oxidative Stability of Silicone Rubber by Incorporating Reduced Graphene Oxide; Impact Factors and Action Mechanism. Polym. Compos. 2018, 39, 1105–1115. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, X.; Zheng, J. Effect of the Sheet Size on the Thermal Stability of Silicone Rubber-Reduced Graphene Oxide Nanocomposites. J. Appl. Polym. Sci. 2019, 136, 47034. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Graphene/Elastomer Nanocomposites. Carbon 2015, 95, 460–484. [Google Scholar] [CrossRef]
- Mensah, B.; Gupta, K.C.; Kim, H.; Wang, W.; Jeong, K.; Nah, C. Graphene-Reinforced Elastomeric Nanocomposites: A review. Polym. Test. 2018, 68, 160–184. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, C.; Santamaría, R.; Granda, M.; Ares, P.; Rodríguez-Reinoso, F.; Menéndez, R. The Effect of the Parent Graphite on the Structure of Graphene Oxide. Carbon 2012, 50, 275–282. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, P.; Granda, M.; Blanco, C.; Santamaría, R.; Romasanta, L.J.; Verdejo, R.; López-Manchado, M.A.; Menéndez, R. Graphene Materials with Different Structures Prepared from the same Graphite by the Hummers and Brodie Methods. Carbon 2013, 65, 156–164. [Google Scholar] [CrossRef]
- Vazquez-Moreno, J.M.; Yuste-Sanchez, V.; Sanchez-Hidalgo, R.; Verdejo, R.; Lopez-Manchado, M.A.; Fernández-García, L.; Blanco, C.; Menéndez, R. Customizing Thermally-Reduced Graphene Oxides for Electrically Conductive or Mechanical Reinforced Epoxy Nanocomposites. Eur. Polym. J. 2017, 93, 1–7. [Google Scholar] [CrossRef]
- Sanchez-Hidalgo, R.; Yuste-Sanchez, V.; Verdejo, R.; Blanco, C.; Lopez-Manchado, M.A.; Menéndez, R. Main Structural Features of Graphene Materials Controlling the Transport Properties of Epoxy Resin-based Composites. Eur. Polym. J. 2018, 101, 56–65. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar]
- Martin-Gallego, M.; Verdejo, R.; Lopez-Manchado, M.A.; Sangermano, M. Epoxy-Graphene UV-Cured Nanocomposites. Polymer 2011, 52, 4664–4669. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University: Ithaca, NY, USA, 1953. [Google Scholar]
- Aguilar-Bolados, H.; Lopez-Manchado, M.A.; Brasero, J.; Avilés, F.; Yazdani-Pedram, M. Effect of the Morphology of Thermally Reduced Graphite Oxide on the Mechanical and Electrical Properties of Natural Rubber Nanocomposites. Compos. Part B 2016, 87, 350–356. [Google Scholar] [CrossRef]
- Dluzneski, P.R. Peroxide Vulcanization of Elastomers. Rubber Chem. Technol. 2001, 74, 451–492. [Google Scholar] [CrossRef]
- Chu, Y.L.; Chen, Y.A.; Li, W.C.; Chu, J.H.; Chen, C.H.; Chiang, C.M. Mechanistic Insights into Light-Driven Graphene-Induced Peroxide Decomposition: Radical Generation and Disproportionation. Chem. Commun. 2016, 52, 9291–9294. [Google Scholar] [CrossRef]
- Meleshevich, A.P. Reactions of Epoxy-Compounds by a Radical Mechanism. Russ. Chem. Rev. 1970, 39, 213. [Google Scholar] [CrossRef]
- Chatterjee, S.; Nafezarefi, F.; Tai, N.H.; Schlagenhauf, L.; Nüesch, F.A.; Chu, B.T.T. Size and Synergy Effects of Nanofiller Hybrids Including Graphene Nanoplatelets and Carbon Nanotubes in Mechanical Properties of Epoxy Composites. Carbon 2012, 50, 5380–5386. [Google Scholar] [CrossRef]
- Martin-Gallego, M.; Hernández, M.; Lorenzo, V.; Verdejo, R.; Lopez-Manchado, M.A.; Sangermano, M. Cationic Photocured Epoxy Nanocomposites Filled with Different Carbon Fillers. Polymer 2012, 53, 1831–1838. [Google Scholar] [CrossRef]
- Toker, D.; Azulay, D.; Shimoni, N.; Balberg, I.; Millo, O. Tunneling and Percolation in Metal-Insulator Composite Materials. Phys. Rev. B Condens. Matter Mater. Phys. 2003, 68, 041403. [Google Scholar] [CrossRef]
- Hernández, M.; Carretero-González, J.; Verdejo, R.; Ezquerra, T.; López-Manchado, M.A. Molecular Dynamics of Natural Rubber/Layered Silicate Nanocomposites as Studied by Dielectric Relaxation Spectroscopy. Macromolecules 2010, 43, 643–651. [Google Scholar] [CrossRef]
- Martin-Gallego, M.; Bernal, M.M.; Hernandez, M.; Verdejo, R.; Lopez-Manchado, M.A. Comparison of Filler Percolation and Mechanical Properties in Graphene and Carbon Nanotubes Filled Epoxy Nanocomposites. Eur. Polym. J. 2013, 49, 1347–1353. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Brasero, J.; Lopez-Manchado, M.A.; Yazdani-Pedram, M. High Performance Natural Rubber/Thermally Reduced Graphite Oxide Nanocomposites by Latex Technology. Compos. Part B 2014, 67, 449–454. [Google Scholar] [CrossRef]
- Yu, A.P.; Ramesh, P.; Sun, X.B.; Bekyarova, E.; Itkis, M.E.; Haddon, R.C. Enhanced Thermal Conductivity in a Hybrid Graphite Nanoplatelet-Carbon Nanotube Filler for Epoxy Composites. Adv. Mater. 2008, 20, 4740–4744. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal Conductivity of Carbon Nanotubes and their Polymer Nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Li, Z.G.; Wu, W.J.; Chen, H.; Zhu, Z.H.; Wang, Y.S.; Zhang, Y. Thermal Conductivity of micro/nano Filler Filled Polymeric Composites. RSC Adv. 2013, 3, 6417–6428. [Google Scholar] [CrossRef]
- Shenogin, S.; Xue, L.; Ozisik, R.; Keblinski, P.; Cahill, D.G. Role of Thermal Boundary Resistance on the Heat Flow in Carbon-Nanotube Composites. J. Appl. Phys. 2004, 95, 8136–8144. [Google Scholar] [CrossRef]
- Song, Y.S.; Youn, J.R. Influence of Dispersion States of Carbon Nanotubes on Physical Properties of Epoxy Nanocomposites. Carbon 2005, 43, 1378–1385. [Google Scholar] [CrossRef]
- Huang, X.; Xie, L.; Jiang, P.; Wang, G.; Liu, F. Electrical, Thermophysical and Micromechanical Properties of Ethylene-Vinyl Acetate Elastomer Composites with Surface Modified BaTiO3 Nanoparticles. J. Phys. D 2009, 42, 245407. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Yu, D.M.; Wang, C.F.; An, Q.L.; Qi, S.H. Effect of Filler Size Distribution on the Mechanical and Physical Properties of Alumina-filled Silicone Rubber. Polym. Eng. Sci. 2008, 48, 1381–1388. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Qi, S.H.; Zhao, H.Z.; Liu, N.L. Thermally Conductive Silicone Rubber Reinforced with Boron Nitride Particle. Polym. Compos. 2007, 28, 23–28. [Google Scholar] [CrossRef]





| Elemental Analysis (wt.%) | XPS (%) | SBET | XRD | Raman Spectroscopy | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | C | O | N | S | H | C/O | Csp2 | Csp3 | C-OH | C-O-C | >C=O | COOH | π-π* | (m2/g) | d002 (nm) | Lc (nm) | La (nm) | n | ID/IG |
| Graphite oxide | 48.2 | 49.3 | 0.1 | 0.3 | 2.1 | 0.98 | 6.0 ± 1.3 | 35.8 ± 5.0 | 6.1 ± 1.2 | 27.7 ± 2.3 | 11.5 ± 5.9 | 13.0 ± 3.6 | 0.0 | 45 | 0.852 | 9.60 | 26.20 | 12 | 1.12 ± 0.03 |
| TRGO-400 | 79.1 | 18.8 | 0.2 | 0.4 | 1.5 | 4.21 | 44.9 ± 1.5 | 25.9 ± 0.6 | 10.1 ± 0.1 | 10.5 ± 0.1 | 3.0 ± 0.1 | 5.5 ± 0.9 | 0.0 | 342 | 0.357 | 1.50 | 7.31 | 4 | 1.29 ± 0.08 |
| TRGO-500 | 81.0 | 17.4 | 0.2 | 0.3 | 1.1 | 4.66 | 46.9 ± 0.4 | 24.1 ± 0.1 | 11.1 ± 0.1 | 9.7 ± 0.4 | 2.5 ± 0.1 | 5.7 ± 0.1 | 0.0 | 400 | 0.357 | 1.75 | 7.31 | 4 | 1.19 ± 0.02 |
| TRGO-700 | 91.0 | 8.0 | 0.3 | 0.0 | 0.7 | 11.38 | 53.9 ± 0.1 | 18.5 ± 6.4 | 10.8 ± 3.0 | 8.8 ± 1.1 | 4.8 ± 1.6 | 3.2 ± 0.9 | 0.0 | 487 | 0.347 | 1.57 | 7.83 | 4 | 1.20 ± 0.02 |
| TRGO-1000 | 98.6 | 1.0 | 0.0 | 0.0 | 0.4 | 98.6 | 67.8 ± 0.3 | 15.1 ± 0.3 | 11.7 ± 1.2 | 0.0 | 4.2 ± 0.2 | 0.0 | 1.2 ± 0.1 | 467 | 0.360 | 1.39 | 8.43 | 5 | 1.48 ± 0.05 |
| TRGO-2000 | 99.7 | 0.2 | 0.0 | 0.0 | 0.1 | 498.5 | 82.7 ± 1.3 | 10.1 ± 0.3 | 5.9 ± 1.3 | 0.0 | 0.0 | 0.0 | 1.3 ± 0.2 | 161 | 0.341 | 6.10 | 11.56 | 19 | 0.18 ± 0.08 |
| Sample | Stress at 50% (MPa) | Stress at 100% (MPa) | Stress at 300% (MPa) | Stress at 500% (MPa) | Maximum Stress (MPa) | Deformation at Break (%) | |
|---|---|---|---|---|---|---|---|
| SR | 0.46 ± 0.01 | 0.66 ± 0.02 | 1.84 ± 0.04 | 3.74 ± 0.10 | 7.28 ± 0.77 | 742 ± 40 | |
| TRGO-400 (phr) | 1 | 0.50 ± 0.02 | 0.76 ± 0.02 | 2.13 ± 0.03 | 4.09 ± 0.04 | 6.60 ± 0.75 | 682 ± 46 |
| 3 | 0.48 ± 0.02 | 0.73 ± 0.02 | 1.68 ± 0.05 | 2.96 ± 0.08 | 5.43 ± 0.38 | 772 ± 26 | |
| 5 | 0.56 ± 0.01 | 0.85 ± 0.01 | 1.72 ± 0.01 | 2.82 ± 0.03 | 4.19 ± 0.17 | 701 ± 27 | |
| TRGO-500 (phr) | 1 | 0.53 ± 0.01 | 0.81 ± 0.01 | 2.27 ± 0.02 | 4.33 ± 0.06 | 7.18 ± 0.67 | 697 ± 39 |
| 3 | 0.83 ± 0.02 | 1.32 ± 0.02 | 3.26 ± 0.05 | 5.47 ± 0.10 | 6.49 ± 0.40 | 579 ± 22 | |
| 5 | 0.77 ± 0.02 | 1.19 ± 0.02 | 2.35 ± 0.02 | 3.69 ± 0.02 | 4.12 ± 0.13 | 559 ± 15 | |
| TRGO-700 (phr) | 1 | 0.52 ± 0.02 | 0.79 ± 0.03 | 2.29 ± 0.06 | 4.46 ± 0.10 | 7.25 ± 0.32 | 689 ± 19 |
| 3 | 1.01 ± 0.03 | 1.61 ± 0.04 | 3.85 ± 0.06 | 6.32 ± 0.03 | 6.17 ± 0.54 | 493 ± 36 | |
| 5 | 1.16 ± 0.02 | 1.80 ± 0.01 | 3.40 ± 0.03 | -- | 3.73 ± 0.14 | 342 ± 19 | |
| TRGO-1000 (phr) | 1 | 0.61 ± 0.02 | 0.94 ± 0.02 | 2.70 ± 0.03 | 5.18 ± 0.08 | 7.28 ± 0.49 | 633 ± 28 |
| 3 | 0.88 ± 0.04 | 1.42 ± 0.06 | 3.77 ± 0.09 | 6.49 ± 0.09 | 6.97 ± 0.35 | 532 ± 23 | |
| 5 | 1.80 ± 0.04 | 3.06 ± 0.06 | -- | -- | 6.00 ± 0.21 | 247 ± 10 | |
| TRGO-2000 (phr) | 1 | 0.50 ± 0.01 | 0.72 ± 0.01 | 1.96 ± 0.04 | 3.86 ± 0.09 | 7.01 ± 0.05 | 710 ± 29 |
| 3 | 0.66 ± 0.02 | 1.07 ± 0.02 | 2.95 ± 0.03 | 5.33 ± 0.03 | 7.48 ± 0.48 | 642 ± 29 | |
| 5 | 0.74 ± 0.02 | 1.25 ± 0.02 | 3.04 ± 0.03 | 4.98 ± 0.03 | 5.99 ± 0.21 | 584 ± 17 | |
| Sample | Smin (dNm) | Smax (dNm) | ΔS (dNm) | ts2 (min) | t90 (min) | Mc (g/mol) | ν (10−4 mol/cm−3) | |
|---|---|---|---|---|---|---|---|---|
| SR | 0.43 | 8.03 | 7.61 | 1.15 | 6.65 | 7305.6 ± 190.5 | 0.68 ± 0.02 | |
| TRGO-400 (phr) | 1 | 0.39 | 7.63 | 7.24 | 1.23 | 7.06 | 6735.2 ± 284.4 | 0.74 ± 0.03 |
| 3 | 0.63 | 7.95 | 7.32 | 1.43 | 13.76 | 6399.0 ± 744.2 | 0.79 ± 0.09 | |
| 5 | 1.38 | 8.43 | 7.05 | 1.35 | 10.63 | 8374.5 ± 55.4 | 0.60 ± 0.01 | |
| TRGO-500 (phr) | 1 | 0.37 | 7.85 | 7.48 | 1.29 | 7.02 | 6335.0 ± 86.1 | 0.79 ± 0.01 |
| 3 | 0.63 | 10.56 | 9.93 | 1.03 | 8.77 | 4733.7 ± 273.4 | 1.06 ± 0.06 | |
| 5 | 1.58 | 10.48 | 8.90 | 1.14 | 8.49 | 5997.2 ± 772.2 | 0.84 ± 0.11 | |
| TRGO-700 (phr) | 1 | 0.39 | 8.31 | 7.92 | 1.05 | 6.87 | 6508.7 ± 31.6 | 0.77 ± 0.01 |
| 3 | 0.80 | 12.44 | 11.64 | 0.94 | 10.00 | 4048.9 ± 113.5 | 1.24 ± 0.03 | |
| 5 | 1.75 | 13.53 | 11.78 | 0.93 | 12.31 | 3981.4 ± 113.5 | 1.26 ± 0.04 | |
| TRGO-1000 (phr) | 1 | 0.53 | 9.67 | 9.14 | 1.09 | 7.83 | 5949.8 ± 237.5 | 0.84 ± 0.03 |
| 3 | 0.78 | 12.54 | 11.76 | 0.85 | 5.95 | 4701.6 ± 111.5 | 1.06 ± 0.03 | |
| 5 | 1.86 | 20.25 | 18.39 | 0.90 | 10.40 | 2342.6 ± 140.1 | 2.07 ± 0.04 | |
| TRGO-2000 (phr) | 1 | 0.48 | 8.55 | 8.07 | 1.15 | 6.34 | 6097.9 ± 660.5 | 0.77 ± 0.01 |
| 3 | 0.56 | 9.83 | 9.27 | 0.95 | 5.75 | 5290.6 ± 227.1 | 0.95 ± 0.04 | |
| 5 | 0.67 | 10.99 | 10.32 | 1.00 | 4.51 | 4701.6 ± 111.5 | 1.06 ± 0.03 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Hidalgo, R.; Blanco, C.; Menendez, R.; Verdejo, R.; Lopez-Manchado, M.A. Multifunctional Silicone Rubber Nanocomposites by Controlling the Structure and Morphology of Graphene Material. Polymers 2019, 11, 449. https://doi.org/10.3390/polym11030449
Sanchez-Hidalgo R, Blanco C, Menendez R, Verdejo R, Lopez-Manchado MA. Multifunctional Silicone Rubber Nanocomposites by Controlling the Structure and Morphology of Graphene Material. Polymers. 2019; 11(3):449. https://doi.org/10.3390/polym11030449
Chicago/Turabian StyleSanchez-Hidalgo, Ruben, Clara Blanco, Rosa Menendez, Raquel Verdejo, and Miguel A. Lopez-Manchado. 2019. "Multifunctional Silicone Rubber Nanocomposites by Controlling the Structure and Morphology of Graphene Material" Polymers 11, no. 3: 449. https://doi.org/10.3390/polym11030449
APA StyleSanchez-Hidalgo, R., Blanco, C., Menendez, R., Verdejo, R., & Lopez-Manchado, M. A. (2019). Multifunctional Silicone Rubber Nanocomposites by Controlling the Structure and Morphology of Graphene Material. Polymers, 11(3), 449. https://doi.org/10.3390/polym11030449