New Poly(lactide-urethane-isocyanurate) Foams Based on Bio-Polylactide Waste
Abstract
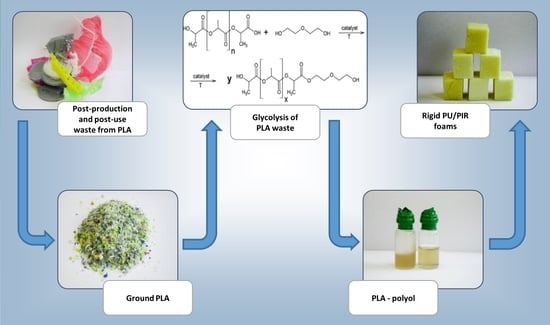
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Glycolysis of 3D Printer PLA
2.3. Examining the Properties of PLA-Polyol
2.3.1. Analytical Tests
2.3.2. Spectroscopy Tests
2.3.3. Differential Scanning Calorimetry (DSC)
2.4. Preparation of RPU/PIR Foams
2.5. Assessing the Properties of RPU/PIR Foams
2.5.1. Processing Times
2.5.2. Selected Properties of New Composites
2.5.3. Flammability Tests
2.5.4. Composites Structure
3. Results and Discussion
3.1. Properties of New PLA-Polyol
3.1.1. Analytical Tests
3.1.2. Spectroscopy Tests
3.1.3. Differential Scanning Calorimetry (DSC)
3.2. Properties of Rigid PU/PIR Foams
3.2.1. Foaming Process
3.2.2. Selected Properties of New Composites
3.2.3. Flammability Tests
3.2.4. Composite Structure
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Plastics—The Facts 2017. An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://www.plasticseurope.org/pl/newsroom/aktualnosci/archiwum-aktualnosci-2018/tworzywa-sztuczne-fakty-2017 (accessed on 24 January 2019).
- Żenkiewicz, M.; Richert, J. Synthesis, properties and applications of polylactide. Przetwórstwo Tworzyw 2009, 5, 192–199. (In Polish) [Google Scholar]
- European Parliament and Council Directive 94/62/EC of 20 December 1994 on Packaging and Packaging Waste; The European Parliament and The Council of The European Union: Copenhagen, Denmark, 1994.
- Stasiek, A.; Dzwonkowski, J.; Łubkowski, D. Studies on extrusion process of biodegradable poly(lactic acid) PLA film. Przetwórstwo Tworzyw 2008, 1, 17. (In Polish) [Google Scholar]
- Malinowski, R.; Richert, S. Triple processing of polylactide affect on mechanical, rheological and thermal properties. Chemik 2010, 64, 246–253. (In Polish) [Google Scholar]
- Nowak, B.; Pająk, J. Biodegradation of poly(lactide) (PLA). Arch. Gospod. Odpadami Ochr. Środowiska, 2010; 12, 1–10. (In Polish) [Google Scholar]
- Murariu, M.; Dechief, A.L.; Ramy-Ratiarison, R.; Paint, Y.; Raquez, J.M.; Dubois, P. Recent advances in production of poly(lactic acid) (PLA) nanocomposites: A versatile method to tune crystallization properties of PLA. Nanocomposites 2015, 1, 71–82. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Moraczewski, K.; Stepczyńska, M.; Malinowski, R.; Budner, B.; Karasiewicz, T.; Jagodziński, B. Selected properties of polylactide containing natural antiaging compounds. Polym. Adv. Technol. 2018, 29, 2963–2971. [Google Scholar] [CrossRef]
- Moraczewski, K.; Stepczyńska, M.; Malinowski, R.; Rytlewski, P.; Jagodziński, B.; Żenkiewicz, M. Stability studies of plasma modification effects of polylactide and polycaprolactone surface layers. Appl. Surf. Sci. 2016, 377, 228–237. [Google Scholar] [CrossRef]
- Gołębiewski, J.; Gibas, E.; Malinowski, R. Selected biodegradable polymers-preparation, properties, applications. Polimery 2008, 53, 799–807. (In Polish) [Google Scholar]
- Malinowski, R. Bioplastics as a new environmentally friendly materials. Inżynieria Ochr. Środowiska 2015, 18, 215–231. (In Polish) [Google Scholar]
- Garrison, T.; Murawski, A.; Quirino, R.L. Bio-based polymers with potential for biodegradability. Polymers 2016, 8, 262. [Google Scholar] [CrossRef]
- Polylactic Acid Properties, Production, Price, Market and Uses. Available online: https://www.plasticsinsight.com/resin-intelligence/resin-prices/polylactic-acid/ (accessed on 25 January 2019).
- Gupta, A.P.; Kumar, V. New emerging trends in synthetic biodegradable polymers—Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Florjańczyk, Z.; Rokicki, G.; Plichta, A.; Parzuchowski, P.; Dębowski, M.; Zychewicz, A.; Lisowska, D. Selected properties of biocomposites from biodegradable polymers with the addition of sewage sludge derived biochar. In Materiały Opakowaniowe z Kompostowalnych Tworzyw Polimerowych; Kowalczuk, M., Żakowska, H., Eds.; Centralny Ośrodek Badawczo-Rozwojowy Opakowań: Warsaw, Poland, 2012; pp. 91–115. ISBN 978-83-60281-11-6. (In Polish) [Google Scholar]
- Duda, A. Poylactide—A polymer of the XXIst century? Przem. Chem. 2003, 82, 905–907. (In Polish) [Google Scholar]
- Wang, X.; Sui, S.; Yan, Y.; Zhang, R. Design and Fabrication of PLGA Sandwiched Cell/Fibrin Constructs for Complex Organ Regeneration. J. Bioact. Compat. Polym. 2010, 25, 229–240. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z. Fabrication and Characterization of Electrospun PLGA/MWNTs/ Hydroxyapatite Biocomposite Scaffolds for Bone Tissue Engineering. J. Bioact. Compat. Polym. 2010, 25, 241–259. [Google Scholar] [CrossRef]
- Malinowski, R.; Łubkowski, D. Changes in polylactide properties during triple reprocessing. Inż. Ap. Chem. 2012, 51, 10–12. (In Polish) [Google Scholar]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Van den Eynde, M.; van Puyvelde, P. 3D Printing of Poly(lactic acid). In Industrial Applications of Poly(Lactic Acid); Advances in Polymer Science; Di Lorenzo, M., Androsch, R., Eds.; Springer: Cham, Switzerland, 2018; Volume 282, pp. 139–158. ISBN 978-3-319-75459-8. [Google Scholar]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Liszkowska, J.; Tomaszewska, E. New polyurethane composites using biodegradable polymer. Inż. Ap. Chem. 2017, 56, 172–173. (In Polish) [Google Scholar]
- Kacperski, M.; Spychaj, T. Rigid polyurethane foams with poly (ethylene terephthalate)/triethanolamine recycling products. Polym. Adv. Technol. 1999, 10, 620–624. [Google Scholar] [CrossRef]
- Čuk, N.; Fabjan, E.; Grželj, P.; Kunaver, M. Water-blown polyurethane/polyisocyanurate foams made from recycled polyethylene terephthalate and liquefied wood-based polyester polyol. J. Appl. Polym. Sci. 2015, 132, 41522. [Google Scholar] [CrossRef]
- Hoang, C.N.; Pham, C.T.; Dang, T.M.; Hoang, D.Q.; Lee, P.-C.; Kang, S.-J.; Kim, J. Novel Oligo-Ester-Ether-Diol Prepared by Waste Poly(ethylene terephthalate) Glycolysis and Its Use in Preparing Thermally Stable and Flame Retardant Polyurethane Foam. Polymers 2019, 11, 236. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Isbrandt, M. Effect of Evening Primrose Oil-Based Polyol on the Properties of Rigid Polyurethane–Polyisocyanurate Foams for Thermal Insulation. Polymers 2018, 10, 1334. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Tomaszewska, E.; Liszkowska, J. Oenothera biennis seed oil as an alternative raw material for production of bio-polyol for rigid polyurethane-polyisocyanurate foams. Ind. Crop. Prod. 2018, 126, 208–217. [Google Scholar] [CrossRef]
- Prociak, A.; Kurańska, M.; Malewska, E. Porous polyurethane plastics synthetized using bio-polyols from renewable raw materials. Polimery 2017, 62, 353–363. [Google Scholar] [CrossRef]
- Seppala, J.; Selin, J.F.; Su, T. Method for Producing Lactic Acid Based Polyurethane. U.S. Patent 5,380,813, 10 January 1995. [Google Scholar]
- Wang, W.; Ping, P.; Chen, X.; Jing, X. Polylactic-based polyurethane and its shape-memory behaviour. Eur. Polym. J. 2006, 42, 1240–1249. [Google Scholar] [CrossRef]
- ASTM International. Standard Practice for Polyurethane Raw Materials: Polyurethane Foam Cup Test; ASTM Standard D7487—13e1, 2008; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Chmiel, E.; Lubczak, J. Oligoetherols and polyurethane foams obtained from metasilicic acid. J. Polym. Bull. 2018, 75, 1579–1596. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Kurańska, M.; Prociak, A.; Malewska, E.; Kulpa, K. Open cell semi-rigid polyurethane foams synthesized using palm oil-based bio-polyol. Ind. Crop. Prod. 2017, 102, 88–96. [Google Scholar] [CrossRef]
- Czupryński, B.; Liszkowska, J.; Paciorek-Sadowska, J. Glycolysis of rigid polyurethane-polyisocyanurate foams. Polimery 2010, 4, 314–319. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A.; Cabulis, U.; Kirpluks, M.; Ryszkowska, J.; Auguścik, M. Innovative porous polyurethane-polyisocyanurate foams based on rapeseed oil and modified with expandable graphite. Ind. Crop. Prod. 2017, 95, 316–323. [Google Scholar] [CrossRef]
- Tu, Y.-C.; Hongyu, F.; Suppes, G.J.; Hsieg, F.-H. Physical properties of water-blown rigid polyurethane foams containing epoxidized soybean oil in different isocyanate indices. J. Appl. Polym. Sci. 2009, 114, 2577–2583. [Google Scholar] [CrossRef]
- Malewska, E.; Prociak, A. The effect of nanosilica filler on the foaming process and properties of flexible polyurethane foams obtained with rapeseed oil-based polyol. Polimery 2015, 60, 472–479. [Google Scholar] [CrossRef]
- Pielichowski, J.; Prociak, A.; Michałowski, S.; Bogdał, D. Application of selected polymeric wastes in the formulation of rigid polyurethane foams. Polimery 2010, 10, 757–764. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Czupryński, B.; Liszkowska, J. Glycolysis of rigid polyurethane-polyisocyanurate foams with reduced flammability. J. Elastomers Plast. 2015, 48, 340–353. [Google Scholar] [CrossRef]
- Król, P.; Żmihorska-Gotfryd, A. Studies on synthesis of oligomeric urethane prepolymers as intermediates to make linear pulyurethanes. Polimery 2000, 72, 1255–1785. (In Polish) [Google Scholar]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Tomaszewska, E.; Liszkowska, J. New bio-polyol based on white mustard seed oil for rigid PUR-PIR foams. Pol. J. Chem. Technol. 2018, 20, 24–31. [Google Scholar] [CrossRef]
- Prociak, A.; Rokicki, G.; Ryszkowska, J. Polyurethane Materials; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2014. (In Polish) [Google Scholar]
- Radziszewska-Zielina, E. Comparative analysis of the parameters of materials used as thermal insulation on external walls. Przegląd Bud. 2009, 80, 32–37. (In Polish) [Google Scholar]
- Paciorek-Sadowska, J. Chemical recycling of rigid PUR-PIR foams with boronitrile flame retardant. Inż. Ap. Chem. 2010, 49, 93–94. [Google Scholar]
- Kozakiewicz, J. Limitations in the use of fluorinated greenhouse gases in the production of polyurethane foams and systems. Polimery 2018, 10, 663–671. [Google Scholar] [CrossRef]
- Chmiel, E.; Lubczak, J.; Stagraczyński, R. Modification of polyurethane foams with 1,3,5-triazine ring and boron. Macromol. Res. 2017, 25, 317–324. [Google Scholar] [CrossRef]
- Lubczak, J.; Łukasiewicz, B.; Myśliwiec, B. Synthesis and applications of oligoetherols with perhydro-1,3,5-triazine ring and boron. J. Appl. Polym. Sci. 2013, 127, 2057–2066. [Google Scholar] [CrossRef]
- Kania, E.; Lubczak, J. Polyurethane Foams with Pyrimidine Rings. Pol. J. Chem. Technol. 2014, 16, 1–6. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.S.; Naik, Y.P. Effect of foam density on the properties of water blown rigid polyurethane foam. J. Appl. Polym. Sci. 2008, 8, 1810–1817. [Google Scholar] [CrossRef]








| Ground PLA (g) | 98% Diethylene Glycol (g) | Zinc Stearate (g) |
|---|---|---|
| 1000.00 | 500.00 | 2.00 |
| Foam Symbol | Rokopol RF-551, (Eq) (g) | PLA Polyol (Eq) (g) | Tegostab 8460 (g) | 33% DABCO (g) | 33% Potassium Acetate (g) | Antiblaze TCMP (g) | Distilled Water (Eq) (g) | Purocyn B (Eq) (g) |
|---|---|---|---|---|---|---|---|---|
| G3.0 | 1.0 66.80 | 0.0 0.00 | 5.40 | 3.15 | 7.95 | 54.00 | 0.7 3.15 | 3.7 250.60 |
| G3.1 | 0.9 60.12 | 0.1 8.60 | 5.42 | 3.19 | 7.96 | 54.22 | 0.7 3.19 | 3.7 250.60 |
| G3.2 | 0.8 53.44 | 0.2 17.21 | 5.45 | 3.21 | 8.03 | 54.54 | 0.7 3.21 | 3.7 250.60 |
| G3.3 | 0.7 46.76 | 0.3 25.81 | 5.49 | 3.23 | 8.08 | 54.87 | 0.7 3.23 | 3.7 250.60 |
| G3.4 | 0.6 40.08 | 0.4 34.42 | 5.52 | 3.25 | 8.13 | 55.20 | 0.7 3.25 | 3.7 250.60 |
| G3.5 | 0.5 33.4 | 0.5 43.02 | 5.55 | 3.27 | 8.18 | 55.53 | 0.7 3.27 | 3.7 250.60 |
| Element | Carbon (%) | Hydrogen (%) | Oxygen (%) |
|---|---|---|---|
| PLA-polyol | 48.31 ± 0.18 | 8.72 ± 0.13 | 42.97 ± 0.21 |
| Foam Symbol | Cream Time (s) | String Gel Time (s) | Tack Free Time (s) | Free Rise Time (s) |
|---|---|---|---|---|
| G3.0 | 10 | 20 | 23 | 40 |
| G3.1 | 12 | 22 | 25 | 42 |
| G3.2 | 12 | 22 | 25 | 42 |
| G3.3 | 12 | 22 | 25 | 42 |
| G3.4 | 12 | 22 | 25 | 42 |
| G3.5 | 12 | 26 | 25 | 42 |
| Parameter | G3.0 | G3.1 | G3.2 | G3.3 | G3.4 | G3.5 |
|---|---|---|---|---|---|---|
| Change of linear dimensions (%) | 1.73 ± 0.18 | 1.29 ± 0.04 | 1.22 ± 0.18 | 1.00 ± 0.11 | 0.80 ± 0.05 | 0.40 ± 0.04 |
| Change of geometric volume (%) | 0.47 ± 0.09 | 1.46 ± 0.17 | 1.91 ± 0.16 | 1.67 ± 0.36 | 1.73 ± 0.14 | 1.39 ± 0.28 |
| Mass loss (%) | 1.00 ± 0.05 | 0.88 ± 0.07 | 1.68 ± 0.23 | 2.28 ± 0.38 | 2.33 ± 0.34 | 3.24 ± 0.39 |
| Parameter | G3.0 | G3.1 | G3.2 | G3.3 | G3.4 | G3.5 |
|---|---|---|---|---|---|---|
| Brittleness (%) | 32.13 ± 2.14 | 16.34 ± 1.03 | 9.59 ± 0.81 | 2.95 ± 0.19 | 1.53 ± 0.18 | 0.60 ± 0.03 |
| Absorbability (%) | 19.42 ± 0.26 | 17.22 ± 0.58 | 16.75 ± 0.81 | 16.73 ± 0.76 | 16.43 ± 0.92 | 16.02 ± 0.76 |
| Water absorption (%) | 5.98 ± 0.61 | 2.25 ± 0.21 | 1.60 ± 0.17 | 1.55 ± 0.10 | 1.42 ± 0.16 | 1.17 ± 0.11 |
| Parameter | G3.0 | G3.1 | G3.2 | G3.3 | G3.4 | G3.5 |
|---|---|---|---|---|---|---|
| Combustion residue (%) | 89.61 ± 0.48 | 91.96 ± 0.21 | 94.97 ± 0.16 | 95.36 ± 0.51 | 97.96 ± 0.63 | 98.04 ± 0.39 |
| LOI (% vol. of O2) | 24.0 ± 0.1 | 24.1 ± 0.1 | 24.1 ± 0.1 | 24.2 ± 0.2 | 24.3 ± 0.1 | 24.3 ± 0.2 |
| Classification based on PN-EN ISO 3582:2002 | self-extinguishing | |||||
| Foam Symbol | Cell Size (μm) | Thickness of Cell Wall (μm) | Content of Cell per Area Unit (cell/mm2) |
|---|---|---|---|
| G3.0 | 213 ± 15 | 17 ± 2 | 13 ± 1 |
| G3.5 | 222 ± 18 | 18 ± 2 | 12 ± 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paciorek-Sadowska, J.; Borowicz, M.; Isbrandt, M. New Poly(lactide-urethane-isocyanurate) Foams Based on Bio-Polylactide Waste. Polymers 2019, 11, 481. https://doi.org/10.3390/polym11030481
Paciorek-Sadowska J, Borowicz M, Isbrandt M. New Poly(lactide-urethane-isocyanurate) Foams Based on Bio-Polylactide Waste. Polymers. 2019; 11(3):481. https://doi.org/10.3390/polym11030481
Chicago/Turabian StylePaciorek-Sadowska, Joanna, Marcin Borowicz, and Marek Isbrandt. 2019. "New Poly(lactide-urethane-isocyanurate) Foams Based on Bio-Polylactide Waste" Polymers 11, no. 3: 481. https://doi.org/10.3390/polym11030481
APA StylePaciorek-Sadowska, J., Borowicz, M., & Isbrandt, M. (2019). New Poly(lactide-urethane-isocyanurate) Foams Based on Bio-Polylactide Waste. Polymers, 11(3), 481. https://doi.org/10.3390/polym11030481