Scaling and Interactions of Linear and Ring Polymer Brushes via DPD Simulations
Abstract
:1. Introduction
2. Model and Methods
2.1. The General DPD Model
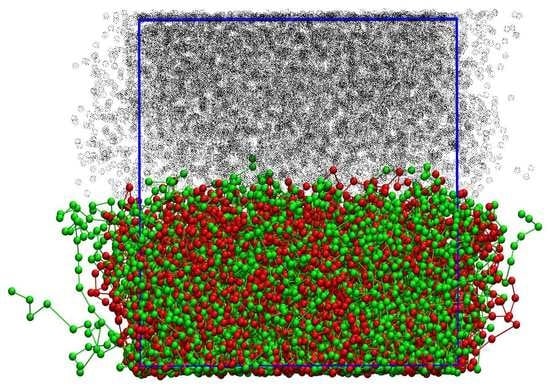
2.2. The Brush Model
2.3. Midpoint Bond Repulsion
3. Simulation Details
4. Results and Discussion
4.1. Statistical Properties of the Brush System
4.2. Decreasing the Distance between Two Brushes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DPD | Dissipative Particle Dynamics |
| mSRP | modified Segmental Repulsion Potential |
References
- Patel, S.S.; Tirrell, M. Measurement of Forces Between Surfaces in Polymer Fluids. Annu. Rev. Phys. Chem. 1989, 40, 597–635. [Google Scholar] [CrossRef]
- Auroy, P.; Auvray, L.; Leger, L. Characterization of the Brush Regime for Grafted Polymer Layers at the Solid-Liquid Interface. Phys. Rev. Lett. 1991, 66, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Perahia, D.; Warburg, S. Forces Between Polymer-Bearing Surfaces Undergoing Shear. Nature 1991, 352, 143–145. [Google Scholar] [CrossRef]
- Klein, J.; Kumacheva, E.; Mahalu, D.; Perahia, D.; Fetters, L.J. Reduction of Frictional Forces Between Solid-Surfaces Bearing Polymer Brushes. Nature 1994, 370, 634–636. [Google Scholar] [CrossRef]
- Klein, J. Shear, Friction, and Lubrication Forces Between Polymer-Bearing Surfaces. Annu. Rev. Mater. Sci. 1996, 26, 581–612. [Google Scholar] [CrossRef]
- Guo, X.; Ballauff, M. Spatial Dimensions of Colloidal Polyelectrolyte Brushes as Determined by Dynamic Light Scattering. Langmuir 2000, 16, 8719–8726. [Google Scholar] [CrossRef]
- Huang, W.X.; Kim, J.B.; Bruening, M.L.; Baker, G.L. Functionalization of Surfaces by Water-Accelerated Atom-Transfer Radical Polymerization of Hydroxyethyl Methacrylate and Subsequent Derivatization. Macromolecules 2002, 35, 1175–1179. [Google Scholar] [CrossRef]
- Minko, S.; Muller, M.; Usov, D.; Scholl, A.; Froeck, C.; Stamm, M. Lateral Versus Perpendicular Segregation in Mixed Polymer Brushes. Phys. Rev. Lett. 2002, 88. [Google Scholar] [CrossRef]
- Raviv, U.; Giasson, S.; Kampf, N.; Gohy, J.F.; Jerome, R.; Klein, J. Lubrication by Charged Polymers. Nature 2003, 425, 163–165. [Google Scholar] [CrossRef]
- Wu, T.; Efimenko, K.; Vlcek, P.; Subr, V.; Genzer, J. Formation and Properties of Anchored Polymers with a Gradual Variation of Grafting Densities on Flat Substrates. Macromolecules 2003, 36, 2448–2453. [Google Scholar] [CrossRef]
- Dunlop, I.E.; Briscoe, W.H.; Titmuss, S.; Jacobs, R.M.J.; Osborne, V.L.; Edmondson, S.; Huck, W.T.S.; Klein, J. Direct Measurement of Normal and Shear Forces between Surface-Grown Polyelectrolyte Layers. J. Phys. Chem. B 2009, 113, 3947–3956. [Google Scholar] [CrossRef] [PubMed]
- Klein, J. Repair or Replacement—A Joint Perspective. Science 2009, 323, 47. [Google Scholar] [CrossRef] [PubMed]
- Klein, J. Hydration Lubrication. Friction 2013, 1, 1–23. [Google Scholar] [CrossRef]
- Alexander, S. Adsorption of Chain Molecules with a Polar Head—A Scaling Description. J. Phys. Fr. 1977, 38, 983–987. [Google Scholar] [CrossRef]
- de Gennes, P.G. Conformations of Polymers Attached to an Interface. Macromolecules 1980, 13, 1069–1075. [Google Scholar] [CrossRef]
- Milner, S.T. Compressing Polymer Brushes—A Quantitative Comparison of Theory and Experiment. Europhys. Lett. 1988, 7, 695–699. [Google Scholar] [CrossRef]
- Milner, S.T.; Witten, T.A.; Cates, M.E. Theory of the Grafted Polymer Brush. Macromolecules 1988, 21, 2610–2619. [Google Scholar] [CrossRef]
- Milner, S.T.; Witten, T.A.; Cates, M.E. Effects of Polydispersity in the End-Grafted Polymer Brush. Macromolecules 1989, 22, 853–861. [Google Scholar] [CrossRef]
- Ball, R.C.; Marko, J.F.; Milner, S.T.; Witten, T.A. Polymers Grafted to a Convex Surface. Macromolecules 1991, 24, 693–703. [Google Scholar] [CrossRef]
- Milner, S.T. Polymer Brushes. Science 1991, 251, 905–914. [Google Scholar] [CrossRef]
- Milner, S.T. Hydrodynamic Penetration Into Parabolic Brushes. Macromolecules 1991, 24, 3704–3705. [Google Scholar] [CrossRef]
- Milner, S.T.; Witten, T.A. Bridging Attraction By Telechelic Polymers. Macromolecules 1992, 25, 5495–5503. [Google Scholar] [CrossRef]
- Netz, R.R.; Schick, M. Classical Theory of Polymer Brushes. Europhys. Lett. 1997, 38, 37–42. [Google Scholar] [CrossRef]
- Netz, R.R.; Schick, M. Polymer Brushes: From Self-Consistent Field Theory to Classical Theory. Macromolecules 1998, 31, 5105–5122. [Google Scholar] [CrossRef] [PubMed]
- Kreer, T.; Muser, M.H.; Binder, K.; Klein, J. Frictional Drag Mechanisms between Polymer-Bearing Surfaces. Langmuir 2001, 17, 7804–7813. [Google Scholar] [CrossRef]
- Zilman, A.G.; Safran, S.A. Entropically Driven Attraction Between Telechelic Brushes. Eur. Phys. J. E 2001, 4, 467–473. [Google Scholar] [CrossRef]
- Currie, E.P.K.; Norde, W.; Stuart, M.A.C. Tethered Polymer Chains: Surface Chemistry and Their Impact on Colloidal and Surface Properties. Adv. Colloid Interface Sci. 2003, 100, 205–265. [Google Scholar] [CrossRef]
- Netz, R.R.; Andelman, D. Neutral and Charged Polymers at Interfaces. Phys. Rep.-Rev. Sect. Phys. Lett. 2003, 380, 1–95. [Google Scholar] [CrossRef]
- Ballauff, M.; Borisov, O. Polyelectrolyte Brushes. Curr. Opin. Colloid Interface Sci. 2006, 11, 316–323. [Google Scholar] [CrossRef]
- Yamamoto, T.; Safran, S.A. Transcription Rates in DNA Brushes. Soft Matter 2015, 11, 3017–3021. [Google Scholar] [CrossRef] [PubMed]
- Kreer, T. Polymer-Brush Lubrication: A Review of Recent Theoretical Advances. Soft Matter 2016, 12, 3479–3501. [Google Scholar] [CrossRef] [PubMed]
- Murat, M.; Grest, G.S. Structure of a Grafted Polymer Brush—A Molecular-Dynamics Simulation. Macromolecules 1989, 22, 4054–4059. [Google Scholar] [CrossRef]
- Lai, P.Y.; Binder, K. Structure and Dynamics Of Polymer Brushes Near The Theta Point—A Monte-Carlo Simulation. J. Chem. Phys. 1992, 97, 586–595. [Google Scholar] [CrossRef]
- Lai, P.Y.; Binder, K. Grafted Polymer Layers Under Shear - A Monte-Carlo Simulation. J. Chem. Phys. 1993, 98, 2366–2375. [Google Scholar] [CrossRef]
- Grest, G.S. Grafted Polymer Brushes—A Constant Surface Pressure Molecular-Dynamics Simulation. Macromolecules 1994, 27, 418–426. [Google Scholar] [CrossRef]
- Grest, G.S. Computer Simulations of Shear and Friction Between Polymer Brushes. Curr. Opin. Colloid Interface Sci. 1997, 2, 271–277. [Google Scholar] [CrossRef]
- Grest, G.S. Normal and Shear Forces Between Polymer Brushes. In Polymers in Confined Environments; Granick, S., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 138, pp. 149–183. [Google Scholar]
- Binder, K. Scaling Concepts for Polymer Brushes and Their Test With Computer Simulation. Eur. Phys. J. E 2002, 9, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Daoulas, K.C.; Terzis, A.F.; Mavrantzas, V.G. Detailed atomistic Monte Carlo simulation of grafted polymer melts. I. Thermodynamic and conformational properties. J. Chem. Phys. 2002, 116, 11028–11038. [Google Scholar] [CrossRef]
- Karayiannis, N.C.; Mavrantzas, V.G.; Theodorou, D.N. A Novel Monte Carlo Scheme for the Rapid Equilibration of Atomistic Model Polymer Systems of Precisely Defined Molecular Architecture. Phys. Rev. Lett. 2002, 88. [Google Scholar] [CrossRef] [PubMed]
- Wenning, L.; Muller, M.; Binder, K. How does the Pattern of Grafting Points Influence the Structure of One-Component and Mixed Polymer Brushes? Europhys. Lett. 2005, 71, 639–645. [Google Scholar] [CrossRef]
- Pastorino, C.; Binder, K.; Kreer, T.; Muller, M. Static and Dynamic Properties of the Interface Between a Polymer Brush and a Melt of Identical Chains. J. Chem. Phys. 2006, 124. [Google Scholar] [CrossRef]
- Alexiadis, O.; Harmandaris, V.A.; Mavrantzas, V.G.; Site, L.D. Atomistic Simulation of Alkanethiol Self-Assembled Monolayers on Different Metal Surfaces via a Quantum, First-Principles Parametrization of the Sulfur Metal Interaction. J. Phys. Chem. C 2007, 111, 6380–6391. [Google Scholar] [CrossRef]
- Dimitrov, D.I.; Milchev, A.; Binder, K. Polymer Brushes in Solvents of Variable Quality: Molecular Dynamics Simulations Using Explicit Solvent. J. Chem. Phys. 2007, 127. [Google Scholar] [CrossRef]
- Hoy, R.S.; Grest, G.S. Entanglements of an End-Grafted Polymer Brush in a Polymeric Matrix. Macromolecules 2007, 40, 8389–8395. [Google Scholar] [CrossRef]
- Pastorino, C.; Kreer, T.; Muller, M.; Binder, K. Comparison of Dissipative Particle Dynamics and Langevin Thermostats for Out-Of-Equilibrium Simulations of Polymeric Systems. Phys. Rev. E 2007, 76. [Google Scholar] [CrossRef]
- Coluzza, I.; Hansen, J.P. Transition from Highly to Fully Stretched Polymer Brushes in Good Solvent. Phys. Rev. Lett. 2008, 100. [Google Scholar] [CrossRef] [Green Version]
- Binder, K.; Kreer, T.; Milchev, A. Polymer Brushes Under Flow and in Other Out-Of-Equilibrium Conditions. Soft Matter 2011, 7, 7159–7172. [Google Scholar] [CrossRef]
- Coluzza, I.; Capone, B.; Hansen, J.P. Rescaling of Structural Length Scales for “Soft Effective Segment” Representations of Polymers in Good Solvent. Soft Matter 2011, 7, 5255–5259. [Google Scholar] [CrossRef]
- Reith, D.; Milchev, A.; Virnau, P.; Binder, K. Anomalous Structure and Scaling of Ring Polymer Brushes. EPL (Europhys. Lett.) 2011, 95. [Google Scholar] [CrossRef]
- Binder, K.; Milchev, A. Polymer Brushes on Flat and Curved Surfaces: How Computer Simulations can Help to Test Theories and to Interpret Experiments. J. Polym. Sci. Part B-Polym. Phys. 2012, 50, 1515–1555. [Google Scholar] [CrossRef]
- Reith, D.; Milchev, A.; Virnau, P.; Binder, K. Computer Simulation Studies of Chain Dynamics in Polymer Brushes. Macromolecules 2012, 45, 4381–4393. [Google Scholar] [CrossRef]
- Sirk, T.W.; Slizoberg, Y.R.; Brennan, J.K.; Lisal, M.; Andzelm, J.W. An Enhanced Entangled Polymer Model for Dissipative Particle Dynamics. J. Chem. Phys. 2012, 136, 134903. [Google Scholar] [CrossRef]
- Hoogerbrugge, P.J.; Koelman, J.M.V.A. Simulating Microscopic Hydrodynamic Phenomena with Dissipative Particle Dynamics. EPL (Europhys. Lett.) 1992, 19, 155. [Google Scholar] [CrossRef]
- Español, P.; Warren, P. Statistical Mechanics of Dissipative Particle Dynamics. EPL (Europhys. Lett.) 1995, 30, 191. [Google Scholar] [CrossRef]
- Groot, R.D.; Warren, P.B. Dissipative Particle Dynamics: Bridging the Gap Between Atomistic and Mesoscopic Simulation. J. Chem. Phys. 1997, 107, 4423–4435. [Google Scholar] [CrossRef]
- Narros, A.; Likos, C.N.; Moreno, A.J.; Capone, B. Multi-Blob Coarse Graining for Ring Polymer Solutions. Soft Matter 2014, 10, 9601–9614. [Google Scholar] [CrossRef]
- Zifferer, G.; Preusser, W. Monte Carlo Simulation Studies of the Size and Shape of Ring Polymers. Macromol. Theory Simul. 2001, 10, 397–407. [Google Scholar] [CrossRef]
- Chubak, I.; Locatelli, E.; Likos, C.N. Ring Polymers Are Much Stronger Depleting Agents than Linear Ones. Mol. Phys. 2018, 116, 2911–2926. [Google Scholar] [CrossRef]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jehser, M.; Zifferer, G.; Likos, C.N. Scaling and Interactions of Linear and Ring Polymer Brushes via DPD Simulations. Polymers 2019, 11, 541. https://doi.org/10.3390/polym11030541
Jehser M, Zifferer G, Likos CN. Scaling and Interactions of Linear and Ring Polymer Brushes via DPD Simulations. Polymers. 2019; 11(3):541. https://doi.org/10.3390/polym11030541
Chicago/Turabian StyleJehser, Martin, Gerhard Zifferer, and Christos N. Likos. 2019. "Scaling and Interactions of Linear and Ring Polymer Brushes via DPD Simulations" Polymers 11, no. 3: 541. https://doi.org/10.3390/polym11030541
APA StyleJehser, M., Zifferer, G., & Likos, C. N. (2019). Scaling and Interactions of Linear and Ring Polymer Brushes via DPD Simulations. Polymers, 11(3), 541. https://doi.org/10.3390/polym11030541