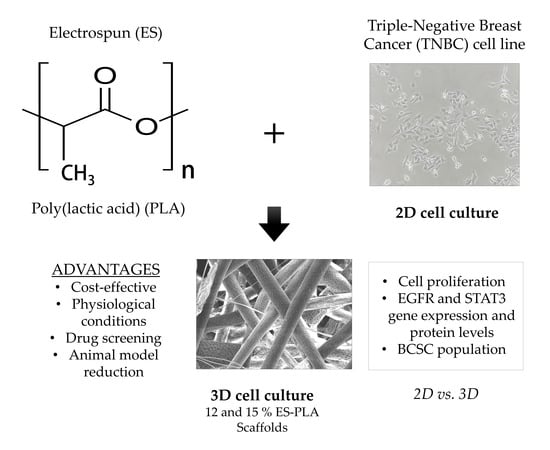
PLA Electrospun Scaffolds for Three-Dimensional Triple-Negative Breast Cancer Cell Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymer
2.2. Polymer Thermal Analysis
2.3. Electrospun Scaffolds Manufacturing
2.4. Scaffold Physical Characterization
2.5. Cell Line and Culture Conditions
2.6. Three-Dimensional Cell Culture
2.7. Cell Proliferation Assay
2.8. Quantitative Real-Time PCR Analysis
2.9. Protein Analysis
2.10. Mammosphere-Forming Assay
2.11. Statistical Analysis
3. Results
3.1. Thermal Characterization of PLA
3.2. Physical Characterization of ES-PLA Scaffolds
3.3. High Proliferation Rates are Obtained in Both 12% and 15% ES-PLA Scaffolds
3.4. EGFR and STAT3 are Altered in 3D-Cultured Cells
3.5. ES-PLA Scaffolds Do Not Increase BCSC Population
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A. Cell culture: Biology’s new dimension. Nature 2003, 424, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Ghajar, C.M.; Bissell, M.J. The need for complex 3D culture models to unravel novel pathways and identify accurate biomarkers in breast cancer. Adv. Drug Deliv. Rev. 2014, 69–70, 42–51. [Google Scholar] [CrossRef] [PubMed]
- DesRochers, T.M.; Palma, E.; Kaplan, D.L. Tissue-engineered kidney disease models. Adv. Drug Deliv. Rev. 2014, 69–70, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Stelzer, E.H.K.; Leicht, S.; Marcello, M. Madin-Darby canine kidney cells are increased in aerobic glycolysis when cultured on flat and stiff collagen-coated surfaces rather than in physiological 3-D cultures. Proteomics 2010, 10, 3394–3413. [Google Scholar] [CrossRef]
- Loessner, D.; Stok, K.S.; Lutolf, M.P.; Hutmacher, D.W.; Clements, J.A.; Rizzi, S.C. Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 2010, 31, 8494–8506. [Google Scholar] [CrossRef]
- Ridky, T.W.; Chow, J.M.; Wong, D.J.; Khavari, P.A. Invasive three-dimensional organotypic neoplasia from multiple normal human epithelia. Nat. Med. 2010, 16, 1450–1455. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-L.; Tian, T.; Nan, K.-J.; Zhao, N.; Guo, Y.-H.; Cui, J.; Wang, J.; Zhang, W.-G. Survival advantages of multicellular spheroids vs. monolayers of HepG2 cells in vitro. Oncol. Rep. 2008, 20, 1465–1471. [Google Scholar] [CrossRef]
- Chopra, V.; Dinh, T.V.; Hannigan, E.V. Three-dimensional endothelial-tumor epithelial cell interactions in human cervical cancers. In Vitro Cell. Dev. Biol. Anim. 1997, 33, 432–442. [Google Scholar] [CrossRef]
- Polonio-Alcalá, E.; Rabionet, M.; Ruiz-Martínez, S.; Ciurana, J.; Puig, T. Three-Dimensional Manufactured Supports for Breast Cancer Stem Cell Population Characterization. Curr. Drug Targets 2018, 20. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Knight, E.; Przyborski, S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J. Anat. 2014, 1–11. [Google Scholar] [CrossRef]
- Sun, Y.; Shan, H.; Wang, J.; Wang, X.; Yang, X.; Ding, J. Laden Nanofiber Capsules for Local Malignancy Chemotherapy. J. Biomed. Nanotechnol. 2019, 15, 939–950. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Xu, H.; Ding, J.-X.; Zhuang, X.-L.; Chen, X.-S.; Chang, F.; Xu, J.-Z.; Li, Z.-M. Molecular weight-modulated electrospun poly(ε-caprolactone) membranes for postoperative adhesion prevention. RSC Adv. 2014, 4, 41696–41704. [Google Scholar] [CrossRef]
- Liu, X.; Holzwarth, J.M.; Ma, P.X. Functionalized Synthetic Biodegradable Polymer Scaffolds for Tissue Engineering. Macromol. Biosci. 2012, 12, 911–919. [Google Scholar] [CrossRef] [Green Version]
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A.; Hošek, J. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef]
- Tamai, H.; Igaki, K.; Kyo, E.; Kosuga, K.; Kawashima, A.; Matsui, S.; Komori, H.; Tsuji, T.; Motohara, S.; Uehata, H. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation 2000, 102, 399–404. [Google Scholar] [CrossRef]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA. Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef]
- Anders, C.K.; Carey, L.A. Biology, Metastatic Patterns, and Treatment of Patients with Triple-Negative Breast Cancer. Clin. Breast Cancer 2009, 9, S73–S81. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, C.; Rody, A.; Gluz, O.; Baumann, K.; Beyer, D.; Kohls, E.-B.; Lausen, K.; Hanker, L.; Holtrich, U.; Becker, S.; et al. The prognostic impact of age in different molecular subtypes of breast cancer. Breast Cancer Res. Treat. 2015, 152, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Cinkaya, A.; Akin, M.; Sengul, A. Evaluation of treatment outcomes of triple-negative breast cancer. J. Cancer Res. Ther. 2016, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.J.; Cano, P.; Rabionet, M.; Puig, T.; Ciurana, J. Effects of different sterilization processes on the properties of a novel 3D-printed polycaprolactone stent. Polym. Adv. Technol. 2018, 29, 2327–2335. [Google Scholar] [CrossRef]
- Rabionet, M.; Yeste, M.; Puig, T.; Ciurana, J. Electrospinning PCL scaffolds manufacture for three-dimensional breast cancer cell culture. Polymers (Basel). 2017, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Giró-Perafita, A.; Palomeras, S.; Lum, D.H.; Blancafort, A.; Viñas, G.; Oliveras, G.; Pérez-Bueno, F.; Sarrats, A.; Welm, A.L.; Puig, T. Preclinical Evaluation of Fatty Acid Synthase and EGFR Inhibition in Triple Negative Breast Cancer. Clin. Cancer Res. 2016, 22, 4687–4697. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real- Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. METHODS 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Giró-Perafita, A.; Rabionet, M.; Puig, T.; Ciurana, J. Optimization of Poli(Ɛ-caprolactone) scaffolds suitable for 3D cancer cell culture. Procedia CIRP 2016, 49, 61–66. [Google Scholar]
- Howes, A.L.; Richardson, R.D.; Finlay, D.; Vuori, K. 3-Dimensional culture systems for anti-cancer compound profiling and high-throughput screening reveal increases in EGFR inhibitor-mediated cytotoxicity compared to monolayer culture systems. PLoS ONE 2014, 9, e108283. [Google Scholar] [CrossRef]
- Leslie, K.; Gao, S.P.; Berishaj, M.; Podsypanina, K.; Ho, H.; Ivashkiv, L.; Bromberg, J. Differential interleukin-6/Stat3 signaling as a function of cellular context mediates Ras-induced transformation. Breast Cancer Res. 2010, 12, R80. [Google Scholar] [CrossRef]
- Polonio-Alcalá, E.; Rabionet, M.; Guerra, A.; Yeste, M.; Ciurana, J.; Puig, T.; Polonio-Alcalá, E.; Rabionet, M.; Guerra, A.J.; Yeste, M.; et al. Screening of Additive Manufactured Scaffolds Designs for Triple Negative Breast Cancer 3D Cell Culture and Stem-Like Expansion. Int. J. Mol. Sci. 2018, 19, 3148. [Google Scholar] [CrossRef] [PubMed]
- Palomeras, S.; Ruiz-Martínez, S.; Puig, T. Targeting Breast Cancer Stem Cells to Overcome Treatment Resistance. Molecules 2018, 23, 2193. [Google Scholar] [CrossRef] [PubMed]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Iinuma, H.; Aoyagi, Y.; Shibuya, H.; Watanabe, T. Predictive value of cancer stem-like cells and cancer-associated genetic markers for peritoneal recurrence of colorectal cancer in patients after curative surgery. Oncology 2010, 78, 309–315. [Google Scholar] [CrossRef]
- Dontu, G.; Abdallah, W.M.; Foley, J.M.; Jackson, K.W.; Clarke, M.F.; Kawamura, M.J.; Wicha, M.S. In vitro propagation and transcriptional profiling of human mammary stem / progenitor cells. Genes Dev. 2003, 17, 1253–1270. [Google Scholar] [CrossRef]
- Okumura-Nakanishi, S.; Saito, M.; Niwa, H.; Ishikawa, F. Oct-3/4 and Sox2 Regulate Oct-3/4 Gene in Embryonic Stem Cells. J. Biol. Chem. 2005, 280, 5307–5317. [Google Scholar] [CrossRef]
- Kotiyal, S.; Bhattacharya, S. Breast cancer stem cells, EMT and therapeutic targets. Biochem. Biophys. Res. Commun. 2014, 453, 112–116. [Google Scholar] [CrossRef]
- Luo, M.; Brooks, M.; Wicha, M.S. Epithelial-mesenchymal plasticity of breast cancer stem cells: Implications for metastasis and therapeutic resistance. Curr. Pharm. Des. 2015, 21, 1301–1310. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.-J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Bueno, G.; Portillo, F.; Cano, A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene 2008, 27, 6958–6969. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Farach-Carson, M.C.; Jia, X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol. Adv. 2014, 32, 1256–1268. [Google Scholar] [CrossRef] [Green Version]
- Palomeras, S.; Rabionet, M.; Ferrer, I.; Sarrats, A.; Garcia-Romeu, M.L.; Puig, T.; Ciurana, J. Breast Cancer Stem Cell Culture and Enrichment Using Poly(ϵ-Caprolactone) Scaffolds. Molecules 2016, 21, 537. [Google Scholar] [CrossRef]
- Feng, S.; Duan, X.; Lo, P.-K.; Liu, S.; Liu, X.; Chen, H.; Wang, Q. Expansion of breast cancer stem cells with fibrous scaffolds. Integr. Biol. (Camb). 2013, 5, 768–777. [Google Scholar] [CrossRef] [Green Version]
- Zou, W.; Chen, R.; Zhang, H.; Qu, J. Preparation, melting behavior and thermal stability of poly(lactic acid)/poly(propylene carbonate) blends processed by vane extruder. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016; Volume 1713, p. 050003. [Google Scholar]
- Damanik, F.F.R.; Spadolini, G.; Rotmans, J.; Farè, S.; Moroni, L. Biological activity of human mesenchymal stromal cells on polymeric electrospun scaffolds. Biomater. Sci. 2019. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D. Beaded nanofibers formed during electrospinning. Polymer (Guildf). 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Chen, M.; Patra, P.K.; Warner, S.B.; Bhowmick, S. Optimization of electrospinning process parameters for tissue engineering scaffolds. Biophys. Rev. Lett. 2006, 01, 153–178. [Google Scholar] [CrossRef]
- Li, S.; Lao, J.; Chen, B.P.; Li, Y.S.; Zhao, Y.; Chu, J.; Chen, K.D.; Tsou, T.C.; Peck, K.; Chien, S. Genomic analysis of smooth muscle cells in 3-dimensional collagen matrix. FASEB J. 2003, 17, 97–99. [Google Scholar] [CrossRef]
- Citri, A.; Yarden, Y. EGF–ERBB signalling: Towards the systems level. Nat. Rev. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Hsu, F.D.; Jensen, K.; Cheang, M.; Karaca, G.; Hu, Z.; Hernandez-Boussard, T.; Livasy, C.; Cowan, D.; Dressler, L.; et al. Immunohistochemical and Clinical Characterization of the Basal-Like Subtype of Invasive Breast Carcinoma. Clin. Cancer Res. 2004, 10, 5367–5374. [Google Scholar] [CrossRef] [Green Version]
- Ekert, J.E.; Johnson, K.; Strake, B.; Pardinas, J.; Jarantow, S.; Perkinson, R.; Colter, D.C. Three-dimensional lung tumor microenvironment modulates therapeutic compound responsiveness in vitro--implication for drug development. PLoS ONE 2014, 9, e92248. [Google Scholar] [CrossRef]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Kroon, P.; Berry, P.A.; Stower, M.J.; Rodrigues, G.; Mann, V.M.; Simms, M.; Bhasin, D.; Chettiar, S.; Li, C.; Li, P.-K.; et al. JAK-STAT Blockade Inhibits Tumor Initiation and Clonogenic Recovery of Prostate Cancer Stem-like Cells. Cancer Res. 2013, 73, 5288–5298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Liu, A.; Peng, Z.; Lin, H.J.; Li, P.K.; Li, C.; Lin, J. STAT3 Is Necessary for Proliferation and Survival in Colon Cancer-Initiating Cells. Cancer Res. 2011, 71, 7226–7237. [Google Scholar] [CrossRef] [PubMed]
- Tomaskovic-Crook, E.; Thompson, E.W.; Thiery, J.P. Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res. 2009, 11, 213. [Google Scholar] [CrossRef]







| Molecular Weight (g/mol) | Melting Point (°C) | Glass Transition (°C) | Young’s Modulus (MPa) | Strain at Break (%) | Degradation Time (Months) |
|---|---|---|---|---|---|
| 30,000 | 173–178 | 60–65 | 108 | 3.5% | 12 |
| Gene | Forward sequence (5′-3′) | Reverse sequence (5′-3′) |
|---|---|---|
| SNAIL | GCTGCAGGACTCTAATCCAGA | ATCTCCGGAGGTGGGATG |
| SOx2 | AACCCCAAGATGCACAACTC | GCTTAGCCTCGTCGATGAAC |
| E-cadh | TGGAGGAATTCTTGCTTTGC | CGCTCTCCTCCGAAGAAAC |
| VIM | TGGTCTAACGGTTTCCCCTA | GACCTCGGAGCGAGAGTG |
| EGFR | CATGTCGATGGACTTCCAGA | GGGACAGCTTGGATCACACT |
| STAT3 | CACCTTCAGGATGTCCGGAA | ATCCTGGAGATTCTCTACCACTTTCA |
| GAPDH | TCTTCCAGGAGCGAGATC | CAGAGATGATGACCCTTTTG |
| Scaffold trait | 12% | 15% |
|---|---|---|
| Weight (mg) | 5.83 ± 0.63 | 6.23 ± 0.44 |
| Thickness (µm) | 68.33 ± 6.89 | 68.00 ± 3.51 |
| 12% PLA | Side | Magnification | ||
| 200× | 1500× | 5000× | ||
| Top |  |  |  | |
| Bottom |  |  |  | |
| 15% PLA | Top |  |  |  |
| Bottom |  |  |  | |
| Scale bars: 150 µm | Scale bars: 20 µm | Scale bars: 6 µm | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polonio-Alcalá, E.; Rabionet, M.; Gallardo, X.; Angelats, D.; Ciurana, J.; Ruiz-Martínez, S.; Puig, T. PLA Electrospun Scaffolds for Three-Dimensional Triple-Negative Breast Cancer Cell Culture. Polymers 2019, 11, 916. https://doi.org/10.3390/polym11050916
Polonio-Alcalá E, Rabionet M, Gallardo X, Angelats D, Ciurana J, Ruiz-Martínez S, Puig T. PLA Electrospun Scaffolds for Three-Dimensional Triple-Negative Breast Cancer Cell Culture. Polymers. 2019; 11(5):916. https://doi.org/10.3390/polym11050916
Chicago/Turabian StylePolonio-Alcalá, Emma, Marc Rabionet, Xavier Gallardo, David Angelats, Joaquim Ciurana, Santiago Ruiz-Martínez, and Teresa Puig. 2019. "PLA Electrospun Scaffolds for Three-Dimensional Triple-Negative Breast Cancer Cell Culture" Polymers 11, no. 5: 916. https://doi.org/10.3390/polym11050916
APA StylePolonio-Alcalá, E., Rabionet, M., Gallardo, X., Angelats, D., Ciurana, J., Ruiz-Martínez, S., & Puig, T. (2019). PLA Electrospun Scaffolds for Three-Dimensional Triple-Negative Breast Cancer Cell Culture. Polymers, 11(5), 916. https://doi.org/10.3390/polym11050916