Influence of Surface Modified Nanodiamonds on Dielectric and Mechanical Properties of Silicone Composites
Abstract
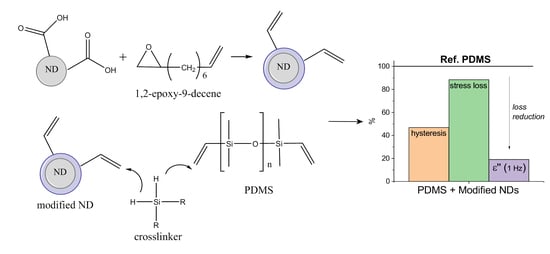
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanodiamond Treatment
2.3. Composite Preparation
2.4. Characterization
3. Results and Discussion
3.1. Surface Modification of Nanodiamonds
3.2. Nanodiamond-Silicone Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dolmatov, V.; Fujimura, T. Physical and Chemical Problems of Modification of Detonation Nanodiamond Surface Properties. Synth. Prop. Appl. Ultrananocrystalline Diam. 2005, 192, 217–230. [Google Scholar]
- Krueger, A. The structure and reactivity of nanoscale diamond. J. Mater. Chem. 2008, 18, 1485–1492. [Google Scholar] [CrossRef]
- Zhang, Y.; Rhee, K.Y.; Hui, D.; Park, S.-J. A critical review of nanodiamond based nanocomposites: Synthesis, properties and applications. Compos. Part B Eng. 2018, 143, 19–27. [Google Scholar] [CrossRef]
- Shakun, A.; Vuorinen, J.; Hoikkanen, M.; Poikelispää, M.; Das, A. Hard nanodiamonds in soft rubbers: Past, present and future – A review. Compos. Part A Appl. Sci. Manuf. 2014, 64, 49–69. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Gogotsi, Y. Nanodiamond–polymer composites. Diamond Related Mater. 2015, 58, 161–171. [Google Scholar] [CrossRef]
- Shakun, A.; Poikelispää, M.; Das, A.; Vuorinen, J. Improved electromechanical response in acrylic rubber by different carbon-based fillers. Polym. Eng. Sci. 2018, 58, 395–404. [Google Scholar] [CrossRef]
- Shakun, A.; Sarlin, E.; Vuorinen, J. Natural rubber-nanodiamond films for the minimisation of losses in dielectric energy harvesters. Int. J. Exergy 2018, 26, 170. [Google Scholar] [CrossRef]
- Hoffstadt, T.; Graf, C.; Maas, J. Optimization of the energy harvesting control for dielectric elastomer generators. Smart Mater. Struct. 2013, 22, 94028. [Google Scholar] [CrossRef]
- Chiba, S.; Waki, M.; Wada, T.; Hirakawa, Y.; Masuda, K.; Ikoma, T. Consistent ocean wave energy harvesting using electroactive polymer (dielectric elastomer) artificial muscle generators. Appl. Energy 2013, 104, 497–502. [Google Scholar] [CrossRef]
- Kornbluh, R.D.; Pelrine, R.; Prahlad, H.; Wong-Foy, A.; McCoy, B.; Kim, S.; Eckerle, J.; Low, T. From Boots to Buoys: Promises and Challenges of Dielectric Elastomer Energy Harvesting; Springer Science and Business Media LLC: Berlin, Germany, 2012; pp. 67–93. [Google Scholar]
- Kaltseis, R.; Keplinger, C.; Koh, S.J.A.; Baumgartner, R.; Goh, Y.F.; Ng, W.H.; Kogler, A.; Tröls, A.; Foo, C.C.; Suo, Z.; et al. Natural rubber for sustainable high-power electrical energy generation. RSC Adv. 2014, 4, 27905–27913. [Google Scholar] [CrossRef] [Green Version]
- Ahnert, K.; Abel, M.; Kollosche, M.; Jørgensen, P.J.; Kofod, G. Soft capacitors for wave energy harvesting. J. Mater. Chem. 2011, 21, 14492–14497. [Google Scholar] [CrossRef] [Green Version]
- Jean-Mistral, C.; Basrour, S. Scavenging Energy from Human Motion with Tubular Dielectric Polymer. Available online: http://dx.doi.org/10.1117/12 (accessed on 29 June 2019).
- Kang, G.; Kim, K.-S.; Kim, S. Note: Analysis of the efficiency of a dielectric elastomer generator for energy harvesting. Rev. Sci. Instruments 2011, 82, 46101. [Google Scholar] [CrossRef] [PubMed]
- Madsen, F.B.; Yu, L.; Daugaard, A.E.; Hvilsted, S.; Skov, A.L. Silicone elastomers with high dielectric permittivity and high dielectric breakdown strength based on dipolar copolymers. Polymer 2014, 55, 6212–6219. [Google Scholar] [CrossRef]
- Madsen, P.J.; Yu, L.; Boucher, S.; Skov, A.L. Enhancing the electro-mechanical properties of polydimethylsiloxane elastomers through blending with poly(dimethylsiloxane-co-methylphenylsiloxane) copolymers. RSC Adv. 2018, 8, 23077–23088. [Google Scholar] [CrossRef]
- Graf, C.; Hitzbleck, J.; Feller, T. Dielectric elastomer–based energy harvesting: Material, generator design, and optimization. J. Intelligent Mater. System Struct. 2014, 25, 951–966. [Google Scholar] [CrossRef]
- Baidakova, M.; Vul’, A. New prospects and frontiers of nanodiamond clusters. J. Phys. D Appl. Phys. 2007, 40, 6300–6311. [Google Scholar] [CrossRef]
- Shenderova, O.; Jones, C.; Borjanovic, V.; Hens, S.; Cunningham, G.; Moseenkov, S.; Kuznetsov, V.; McGuire, G. Detonation nanodiamond and onion-like carbon: applications in composites. Phys. Status Solidi (a) 2008, 205, 2245–2251. [Google Scholar] [CrossRef]
- Voznyakovski, A.P.; Kudoyarov, M.F.; Pozdnyakov, O.F. Self-organization and sedimentation stability of detonation nanodiamond suspensions. Tech. Phys. Lett. 2007, 33, 29–36. [Google Scholar] [CrossRef]
- Kong, S.; Mariatti, M.; Busfield, J. Effects of types of fillers and filler loading on the properties of silicone rubber composites. J. Reinf. Plast. Compos. 2011, 30, 1087–1096. [Google Scholar] [CrossRef]
- Neverovskaya, A.Y.; Voznyakovskii, A.P.; Dolmatov, V.Y. Structure of the dispersive medium and sedimentation resistance of suspensions of detonation nanodiamonds. Phys. Solid State 2004, 46, 662–664. [Google Scholar] [CrossRef]
- Schrand, A.M.; Hens, S.A.C.; Shenderova, O.A. Nanodiamond Particles: Properties and Perspectives for Bioapplications. Crit. Rev. Solid State Mater. Sci. 2009, 34, 18–74. [Google Scholar] [CrossRef]
- Krueger, A. Chapter 8 - current issues and challenges in surface chemistry of nanodiamonds. In Nanodiamonds; Arnault, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 183–242. [Google Scholar]
- Brochier Salon, M.; Bayle, P.; Abdelmouleh, M.; Boufi, S.; Belgacem, M.N. Kinetics of hydrolysis and self condensation reactions of silanes by NMR spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 2008, 312, 83–91. [Google Scholar] [CrossRef]
- Altmann, S.; Pfeiffer, J. The Hydrolysis/Condensation Behaviour of Methacryloyloxyalkylfunctional Alkoxysilanes: Structure-Reactivity Relations. Monatshefte Chemie-Chemical Monthly 2003, 134, 1081–1092. [Google Scholar] [CrossRef]
- Krueger, A.; Williams, O.A. (Eds.) The Chemistry of Nanodiamond. Royal Society of Chemistry; No. 2014-January; RSC Nanoscience and Nanotechnology: Cambridge, UK, 2014. [Google Scholar]
- Krüger, A.; Liang, Y.; Jarre, G.; Stegk, J.; Kr?ger, A. Surface functionalisation of detonation diamond suitable for biological applications. J. Mater. Chem. 2006, 16, 2322–2328. [Google Scholar] [CrossRef]
- Edgington, R.; Spillane, K.M.; Papageorgiou, G.; Wray, W.; Ishiwata, H.; Labarca, M.; Leal-Ortiz, S.; Reid, G.; Webb, M.; Foord, J.; et al. Functionalisation of Detonation Nanodiamond for Monodispersed, Soluble DNA-Nanodiamond Conjugates Using Mixed Silane Bead-Assisted Sonication Disintegration. Sci. Rep. 2018, 8, 728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajiali, F.; Shojaei, A. Silane functionalization of nanodiamond for polymer nanocomposites-effect of degree of silanization. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 506, 254–263. [Google Scholar] [CrossRef]
- Neburkova, J.; Vavra, J.; Cigler, P. Coating nanodiamonds with biocompatible shells for applications in biology and medicine. Curr. Opin. Solid State Mater. Sci. 2017, 21, 43–53. [Google Scholar] [CrossRef]
- Li, Z.; Tatsuya, T.; Masaaki, I.; Naoko, K.; Takahide, K.; Naoki, K. Chromatographic Separation of Highly Soluble Diamond Nanoparticles Prepared by Polyglycerol Grafting. Angew. Chem. Int. Ed. 2011, 50, 1388–1392. [Google Scholar]
- Huang, Z.; Ji, H.; Mays, J.W.; Dadmun, M.D. Understanding the Grafting of Telechelic Polymers on a Solid Substrate to Form Loops. Macromolecules 2008, 41, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Blank, W.J.; He, Z.A.; Picci, M. Catalysis of the epoxy-carboxyl reaction. J. Coat. Technol. 2002, 74, 33–41. [Google Scholar] [CrossRef]
- Schönherr, J.; Buchheim, J.R.; Scholz, P.; Adelhelm, P. Boehm Titration Revisited (Part I): Practical Aspects for Achieving a High Precision in Quantifying Oxygen-Containing Surface Groups on Carbon Materials. C 2018, 4, 21. [Google Scholar] [CrossRef]
- Tiwari, M.; Datta, R.N.; Talma, A.G.; Noordermeer, J.W.M.; Dierkes, W.K.; Van Ooij, W.J. Comparative Study of Plasma-Thiophene and -Acetylene Coated Silica in SBR and EPDM Reinforcement. Rubber Chem. Technol. 2009, 82, 473–491. [Google Scholar] [CrossRef]
- Osswald, S.; Yushin, G.; Mochalin, V.; Kucheyev, S.O.; Gogotsi, Y. Control of sp2/sp3Carbon Ratio and Surface Chemistry of Nanodiamond Powders by Selective Oxidation in Air. J. Am. Chem. Soc. 2006, 128, 11635–11642. [Google Scholar] [CrossRef] [PubMed]
- Bradac, C.; Gaebel, T.; Pakes, C.I.; Say, J.M.; Zvyagin, A.V.; Rabeau, J.R. Effect of the Nanodiamond Host on a Nitrogen-Vacancy Color-Centre Emission State. Small 2013, 9, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Stehlik, S.; Glatzel, T.; Pichot, V.; Pawlak, R.; Meyer, E.; Spitzer, D.; Rezek, B. Water interaction with hydrogenated and oxidized detonation nanodiamonds — Microscopic and spectroscopic analyses. Diam. Relat. Mater. 2016, 63, 97–102. [Google Scholar] [CrossRef]
- Ji, S.; Jiang, T.; Xu, K.; Li, S. FTIR study of the adsorption of water on ultradispersed diamond powder surface. Appl. Surf. Sci. 1998, 133, 231–238. [Google Scholar] [CrossRef]
- Efremov, V.P.; Zakatilova, E.I.; Maklashova, I.V.; Shevchenko, N.V. Thermal stability of detonation-produced micro and nanodiamonds. J. Phys. Conf. Ser. 2018, 946, 012107. [Google Scholar]
- Casalini, R.; Roland, C. Aging of a low molecular weight poly(methyl methacrylate). J. Non-Crystalline Solids 2011, 357, 282–285. [Google Scholar] [CrossRef]
- Roggero, A.; Dantras, E.; Paulmier, T.; Tonon, C.; Lewandowski, S.; Dagras, S.; Payan, D. Dynamic glass transition of filled polysiloxane upon electron irradiation. J. Non-Crystalline Solids 2017, 455, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Glatz-Reichenbach, J.K.W.; Sorriero, L.; Fitzgerald, J.J. Influence of Crosslinking on the Molecular Relaxation of an Amorphous Copolymer Near Its Glass-Transition Temperature. Macromolecules 1994, 27, 1338–1343. [Google Scholar] [CrossRef]
- Dolmatov, V.Y. Polymer—Diamond composites based on detonation nanodiamonds. Part 3. J. Superhard Mater. 2007, 29, 199–205. [Google Scholar] [CrossRef]
- Maus, A.; Saalwächter, K. Crystallization Kinetics of Poly(dimethylsiloxane) Molecular-Weight Blends—Correlation with Local Chain Order in the Melt? Macromol. Chem. Phys. 2007, 208, 2066–2075. [Google Scholar] [CrossRef]









| Surface Groups, mmol/g | ||||
|---|---|---|---|---|
| Carboxyl | Lactone | Phenolic | All Surface Groups | |
| ND reference | 0.131 | 0.101 | 0.219 | 0.451 |
| ND oxidized | 0.329 | 0.018 | 0.127 | 0.475 |
| Sample | Element Content, % | |||
|---|---|---|---|---|
| C | O | Si | Others | |
| ND-COOH | 96.0 | 3.2 | - | <1 |
| ND-VTMS | 54.6 | 18.0 | 27.3 | <1 |
| ND-ED(40C) | 96.0 | 4.0 | - | - |
| ND-GOPTMS | 90.8 | 6.6 | 2.6 | - |
| Sample | Modulus, MPa | Apparent Crosslink Density 1/Q, - | ||
|---|---|---|---|---|
| 10% | 50% | 100% | ||
| PDMS/0 | 0.12 ± 0.01 | 0.49 ± 0.03 | 1.34 ± 0.11 | 0.689 ± 0.005 |
| PDMS_oil/0 | 0.12 ± 0.01 | 0.45 ± 0.01 | 1.38 ± 0.09 | 0.614 ± 0.003 |
| PDMS_oil/COOH_0.1 | 0.11 ± 0.01 | 0.49 ± <0.01 | 1.13 ± 0.22 | 0.683 ± 0.003 |
| PDMS_oil/VTMS_0.1 | 0.10 ± 0.01 | 0.47 ± 0.01 | 1.39 ± 0.02 | 0.674 ± 0.004 |
| PDMS_oil/ED_0.1 | 0.10 ± 0.02 | 0.44 ± 0.01 | 1.38 ± 0.10 | 0.679 ± 0.003 |
| PDMS_oil/GOPTMS_0.1 | 0.11 ± 0.01 | 0.45 ± 0.01 | 1.39 ± 0.03 | 0.554 ± 0.047 |
| Sample | Tg, °C | Enthalpy, J/g | Degree of Crystallinity*, % | |
|---|---|---|---|---|
| Cold Crystallization | Melting | |||
| PDMS/0 | −127.0 | 3.26 | 3.84 | 0.9 |
| PDMS_oil/0 | −127.3 | 0.78 | 0.92 | 0.2 |
| PDMS_oil/COOH_0.1 | −124.8 | 1.45 | 15.37 | 22.7 |
| PDMS_oil/VTMS_0.1 | −120.6 | - | 15.47 | 25.2 |
| PDMS_oil/ED_0.1 | −121.6 | - | 14.90 | 24.3 |
| PDMS_oil/GOPTMS_0.1 | −126.6 | 9.57 | 13.58 | 6.5 |
| Sample | Hysteresis Loss, % | Stress Loss, % | |||
|---|---|---|---|---|---|
| 1st Cycle | 10th Cycle | after 1 min | after 5 min | after 1 h | |
| PDMS/0 | 1.49 ± 0.17 | 0.63 ± 0.20 | 1.08 ± 0.04 | 1.77 ± 0.11 | 2.83 ± 0.47 |
| PDMS_oil/0 | 2.21 ± 0.27 | 0.64 ± 0.15 | 0.78 ± 0.13 | 1.30 ± 0.28 | 2.26 ± 0.38 |
| PDMS_oil/COOH_0.1 | 1.70 ± 0.25 | 0.64 ± 0.37 | 0.61 ± 0.17 | 0.96 ± 0.23 | 1.98 ± 0.38 |
| PDMS_oil/VTMS_0.1 | 1.59 ± 0.66 | 0.46 ± 0.23 | 0.56 ± 0.09 | 0.86 ± 0.06 | 1.47 ± 0.16 |
| PDMS_oil/ED_0.1 | 1.89 ± 0.71 | 0.30 ± 0.10 | 0.69 ± 0.04 | 1.22 ± 0.28 | 2.50 ± 0.41 |
| PDMS_oil/GOPTMS_0.1 | 1.48 ± 0.37 | 0.37 ± 0.18 | 0.62 ± 0.12 | 1.07 ± 0.21 | 2.41 ± 0.37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakun, A.; Anyszka, R.; Sarlin, E.; Blume, A.; Vuorinen, J. Influence of Surface Modified Nanodiamonds on Dielectric and Mechanical Properties of Silicone Composites. Polymers 2019, 11, 1104. https://doi.org/10.3390/polym11071104
Shakun A, Anyszka R, Sarlin E, Blume A, Vuorinen J. Influence of Surface Modified Nanodiamonds on Dielectric and Mechanical Properties of Silicone Composites. Polymers. 2019; 11(7):1104. https://doi.org/10.3390/polym11071104
Chicago/Turabian StyleShakun, Alexandra, Rafal Anyszka, Essi Sarlin, Anke Blume, and Jyrki Vuorinen. 2019. "Influence of Surface Modified Nanodiamonds on Dielectric and Mechanical Properties of Silicone Composites" Polymers 11, no. 7: 1104. https://doi.org/10.3390/polym11071104
APA StyleShakun, A., Anyszka, R., Sarlin, E., Blume, A., & Vuorinen, J. (2019). Influence of Surface Modified Nanodiamonds on Dielectric and Mechanical Properties of Silicone Composites. Polymers, 11(7), 1104. https://doi.org/10.3390/polym11071104