Microstructural Changes of Aramid Fiber Due to Reaction with Toluene 2,4-diisocyanate under Tension in scCO2
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Treatment in scCO2
2.3. Tensile Testing
2.4. Scanning Electron Microscopy
2.5. Thermogravimetric Analysis
2.6. WAXS and SAXS Measurements
2.7. WAXS and SAXS Data Analyses
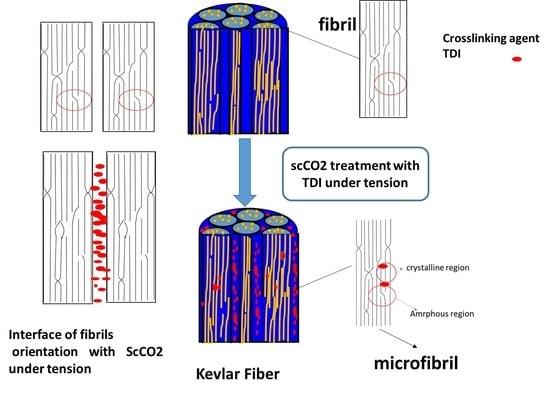
3. Results and Discussion
3.1. The Amorphous Phase of the Kevlar 49 Fiber
3.2. Mechanical Properties of the Kevlar 49 Fibers
3.3. Crystal Structure of a Kevlar 49 Fiber in scCO2 from the WAXS Results
3.4. Fibrillar Structure Evolution from SAXS Measurements
3.5. Thermogravimetric Analysis Results
3.6. Model of Progress
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edmunds, R.; Wadee, M.A. On kink banding in individual PPTA fibres. Compos. Sci. Technol. 2005, 65, 1284–1298. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, Q.; Xing, Z.; Zhang, W.; Zhang, M.; Tan, H.; Wang, J.; Wu, G. Improving the Supercritical CO2 Foaming of Polypropylene by the Addition of Fluoroelastomer as a Nucleation Agent. Polymers 2019, 11, 226. [Google Scholar] [CrossRef] [PubMed]
- Panar, M.; Avakian, P.; Blume, R.C.; Gardner, K.H.; Gierke, T.D.; Yang, H.H. Morphology of poly(p-phenylene terephthalamide) fibers. J. Polym. Sci. Polym. Phys. Ed. 1983, 21, 1955–1969. [Google Scholar] [CrossRef]
- Yang, H.H. Kevlar Aramid Fiber; Wiley and Songs: New York, NY, USA, 1993. [Google Scholar]
- Morgan, R.J.; Pruneda, C.O.; Steele, W.J. The relationship between the physical structure and the microscopic deformation and failure processes of poly(p-phenylene terephthalamide) fibers. Polym. Sci. Polym. Phys. 1983, 21, 1757–1783. [Google Scholar] [CrossRef]
- Mathur, A.; Netravali, A.N. Mechanical Property Modification of Aramid Fibers by Polymer Infiltration. Text. Res. J. 1996, 66, 201–208. [Google Scholar] [CrossRef]
- Litchfield, D.W.; Baird, D.G. The role of nanoclay in the generation of poly(ethylene terephthalate) fibers with improved modulus and tenacity. Polymer 2008, 49, 5027–5036. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, L.; Zhao, H.; Song, G.; Tang, F.; Wang, L.; Shao, H.; Zhang, Y. Lamellar and fibrillar structure evolution of poly(ethylene terephthalate) fiber in thermal annealing. Polymer 2016, 105, 157–166. [Google Scholar] [CrossRef]
- Allen, S.R. Tensile recoil measurement of compressive strength for polymeric high performance fibres. J. Mater. Sci. 1987, 22, 853–859. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Wang, H.; Zhou, G.; Nie, H. High-expanded foams based on novel long-chain branched poly(aryl ether ketone) via ScCO2 foaming method. Polymer 2019, 165, 124–132. [Google Scholar] [CrossRef]
- Dobb, M.G.; Johnson, D.J.; Saville, B.P. Supramolecular structure of a high-modulus polyaromatic fiber (Kevlar 49). J. Polym. Sci. Polym. Phys. Ed. 1977, 15, 2201–2211. [Google Scholar] [CrossRef]
- Kotera, M.; Nakai, A.; Saito, M.; Izu, T.; Nishino, T. Elastic Modulus of the Crystalline Regions of Poly(p-phenylene terephthalamide) Single Fiber Using SPring-8 Synchrotron Radiation. Polym. J. 2007, 39, 1295–1299. [Google Scholar] [CrossRef]
- Li, C.-S.; Zhan, M.-S.; Huang, X.-C.; Zhou, H. The evolution of structure and properties of poly(p-phenylene terephthalamide) during the hydrothermal aging. J. Appl. Polym. Sci. 2012, 126, 552–558. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, S.Z.D.; Geil, P.H. Morphology and crystal structure in single crystals of poly(p-phenylene terephthalamide) prepared by melt polymerization. Polymer 1996, 37, 1413–1430. [Google Scholar] [CrossRef]
- Iqbal, M.; Mensen, C.; Qian, X.; Picchioni, F. Green Processes for Green Products: The Use of Supercritical CO2 as Green Solvent for Compatibilized Polymer Blends. Polymers 2018, 10, 1285. [Google Scholar] [CrossRef] [PubMed]
- Kamali, H.; Mollaee, R.; Khodaverdi, E.; Hadizadeh, F.; Zohuri, G.H. Ring-opening polymerization of poly(d,l-lactide-co-glycolide)-poly(ethylene glycol) diblock copolymer using supercritical CO2. J. Supercrit. Fluids 2019, 145, 133–139. [Google Scholar] [CrossRef]
- de Moura, J.M.L.N.; Gonçalves, L.A.G.; Sarmento, L.A.V.; Petrus, J.C.C. Purification of structured lipids using SCCO2 and membrane process. J. Membrane Sci. 2007, 299, 138–145. [Google Scholar] [CrossRef]
- Zhou, D.; Xiong, Y.; Yuan, H.; Luo, G.; Zhang, J.; Shen, Q.; Zhang, L. Synthesis and compressive behaviors of PMMA microporous foam with multi-layer cell structure. Compos. Part B Eng. 2019, 165, 272–278. [Google Scholar] [CrossRef]
- Zhang, H.-C.; Yu, C.-N.; Liang, Y.; Lin, G.-X.; Meng, C. Foaming Behavior and Microcellular Morphologies of Incompatible SAN/CPE Blends with Supercritical Carbon Dioxide as a Physical Blowing Agent. Polymers 2019, 11, 89. [Google Scholar] [CrossRef]
- Qiao, M.; Kong, H.; Ding, X.; Hu, Z.; Zhang, L.; Cao, Y.; Yu, M. Study on the Changes of Structures and Properties of PAN Fibers during the Cyclic Reaction in Supercritical Carbon Dioxide. Polymers 2019, 11, 402. [Google Scholar] [CrossRef]
- Wolff, S.; Beuermann, S.; Türk, M. Impact of rapid expansion of supercritical solution process conditions on the crystallinity of poly(vinylidene fluoride) nanoparticles. J. Supercrit. Fluids 2016, 117, 18–25. [Google Scholar] [CrossRef]
- Frerich, S.C. Biopolymer foaming with supercritical CO2—Thermodynamics, foaming behaviour and mechanical characteristics. J. Supercrit. Fluids 2015, 96, 349–358. [Google Scholar] [CrossRef]
- Nofar, M. Effects of nano-/micro-sized additives and the corresponding induced crystallinity on the extrusion foaming behavior of PLA using supercritical CO2. Mater Des. 2016, 101, 24–34. [Google Scholar] [CrossRef]
- Vopilov, Y.E.; Nikitin, L.N.; Yurkov, G.Y.; Kharitonova, E.P.; Khokhlov, A.R.; Bouznik, V.M. Effect of supercritical carbon dioxide on ultradispersed polytetrafluoroethylene. J. Supercrit. Fluids 2012, 62, 204–210. [Google Scholar] [CrossRef]
- da Silva, C.V.; Pereira, V.J.; Rosa, P.T.V.; Cabral-Albuquerque, E.C.M.; de Melo, S.A.B.V.; Costa, G.M.N.; Dias, A.M.A.; de Sousa, H.C.; Braga, M.E.M. Effect of scCO2 sorption capacity on the total amount of borage oil loaded by scCO2 impregnation/deposition into a polyurethane-based wound dressing. J. Supercrit. Fluids 2016, 115, 1–9. [Google Scholar] [CrossRef]
- Wang, K.; Pang, Y.; Liu, W.; Wu, F.; Zheng, W. A new approach designed for improving in situ compatibilization of polypropylene/polystyrene blends via reactive extrusion with supercritical CO2 as the processing medium. J. Supercrit. Fluids 2016, 118, 203–209. [Google Scholar] [CrossRef]
- Baseri, S.; Karimi, M.; Morshed, M. Study of structural changes and mesomorphic transitions of oriented poly(ethylene therephthalate) fibers in supercritical CO2. Eur. Polym. J. 2012, 48, 811–820. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Brantley, N.H.; Eckert, C.A. Applications of vibrational spectroscopy to characterize poly(ethylene terephthalate) processed with supercritical CO2. Vib. Spectrosc. 1999, 19, 277–283. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Z.; Yang, L.; Li, X.; Xiang, K. Study on Surface Properties of Aramid Fiber Modified in Supercritical Carbon Dioxide by Glycidyl-POSS. Polymers 2019, 11, 700. [Google Scholar] [CrossRef]
- Qiao, M.; Kong, H.; Ding, X.; Hu, Z.; Zhang, L.; Cao, Y.; Yu, M. Effect of Different Pressures of Supercritical Carbon Dioxide on the Microstructure of PAN Fibers during the Hot-Drawing Process. Polymers 2019, 11, 403. [Google Scholar] [CrossRef]
- Kong, H.; Teng, C.; Liu, X.; Zhou, J.; Zhong, H.; Zhang, Y.; Han, K.; Yu, M. Simultaneously improving the tensile strength and modulus of aramid fiber by enhancing amorphous phase in supercritical carbon dioxide. RSC Adv. 2014, 4, 20599–20604. [Google Scholar] [CrossRef]
- Haijuan, K.; Peng, Y.; Cuiqing, T.; Muhuo, Y. Surface modification of poly(p-phenylene terephthalamide) fibers with HDI assisted by supercritical carbon dioxide. RSC Adv. 2015, 5, 58916–58920. [Google Scholar] [CrossRef]
- Lee, K.G.; Barton, R.; Schultz, J.M. Structure and property development in poly(p-phenylene terephthalamide) during heat treatment under tension. J. Polym. Sci. Part B Polym. Phys. 1995, 33, 1–14. [Google Scholar] [CrossRef]
- Ran, S.; Fang, D.; Zong, X.; Hsiao, B.S.; Chu, B.; Cunniff, P.M. Structural changes during deformation of Kevlar fibers via on-line synchrotron SAXS/WAXD techniques. Polymer 2001, 42, 1601–1612. [Google Scholar] [CrossRef]
- Guinier, A. X-Ray Diffraction in Crystals, Imperfect Crystals, and Amorphous Bodies. Phys. Today 1964, 17, 70–72. [Google Scholar] [CrossRef]
- Grubb, D.T.; Prasad, K.; Adams, W. Small-Angle X-Ray-Diffraction of Kevlar Using Synchrotron Radiation. Polymer 1991, 32, 1167–1172. [Google Scholar] [CrossRef]
- Wang, W.; Ruland, W.; Cohen, Y. Fibrillar and Microfibrillar Structures in Poly(p-Phenylene Terephthalamide) Fibers. Acta Polym. 1993, 44, 273–278. [Google Scholar] [CrossRef]
- John, M.J.; Anandjiwala, R.D. Surface modification and preparation techniques for textile materials. Surf. Modif. Text. 2009, 1–25. [Google Scholar] [CrossRef]
- van der Zwaag, S. Structure and properties of aramid fibres. Dev. Sci. Technol. Compos. Mater. 2009, 1, 394–412. [Google Scholar]
- Itoh, T.; Hashimoto, M. Observation on skin-core, microfibril and pleated band morphology in poly(p-phenylene terephthalamide) fibers using transmission and scanning electron microscopes. Sen’i Gakkaishi 1997, 53, 565–569. [Google Scholar] [CrossRef]












| Tension (CN/dtex) | Misorientation Bφ (o) | Microfibril Length L (nm) |
|---|---|---|
| as-received | 9.7 | 1224.4 |
| 0 | 6.8 | 1118.6 |
| 0.2 | 7.9 | 1150.1 |
| 0.4 | 7.5 | 863.8 |
| 0.6 | 11.4 | 733.4 |
| 0.8 | 16.1 | 936.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, H.; Xu, Q.; Yu, M. Microstructural Changes of Aramid Fiber Due to Reaction with Toluene 2,4-diisocyanate under Tension in scCO2. Polymers 2019, 11, 1110. https://doi.org/10.3390/polym11071110
Kong H, Xu Q, Yu M. Microstructural Changes of Aramid Fiber Due to Reaction with Toluene 2,4-diisocyanate under Tension in scCO2. Polymers. 2019; 11(7):1110. https://doi.org/10.3390/polym11071110
Chicago/Turabian StyleKong, Haijuan, Qian Xu, and Muhuo Yu. 2019. "Microstructural Changes of Aramid Fiber Due to Reaction with Toluene 2,4-diisocyanate under Tension in scCO2" Polymers 11, no. 7: 1110. https://doi.org/10.3390/polym11071110
APA StyleKong, H., Xu, Q., & Yu, M. (2019). Microstructural Changes of Aramid Fiber Due to Reaction with Toluene 2,4-diisocyanate under Tension in scCO2. Polymers, 11(7), 1110. https://doi.org/10.3390/polym11071110