Plasma Nanocoatings Developed to Control the Shear Strength of Polymer Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasma Nanotechnology
2.2. Plasma Nanocoatings Characterization
2.3. Composite Short Beams and Their Shear Strength
3. Results and Discussion
3.1. Selection of Deposition Conditions
3.2. Mechanical, Optical, and Chemical Properties of Plasma Nanocoatings
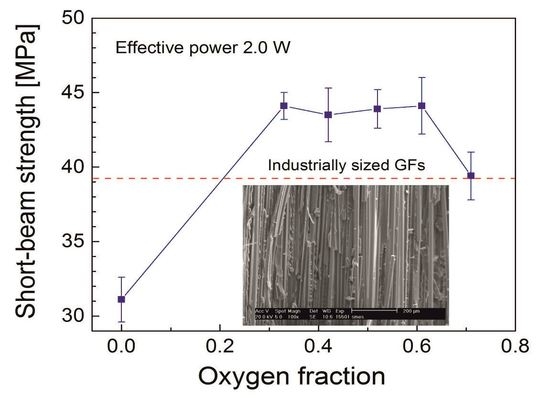
3.3. Shear Properties of Glass-Fiber (GF)/Polyester Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Romera, D.; Couleaud, P.; Mejias, S.H.; Aires, A.; Cortajarena, A.L.; Cortajarena, A. Biomolecular templating of functional hybrid nanostructures using repeat protein scaffolds. Biochem. Soc. Trans. 2015, 43, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Cech, V.; Palesch, E.; Lukes, J. The glass fiber–polymer matrix interface/interphase characterized by nanoscale imaging techniques. Compos. Sci. Technol. 2013, 83, 22–26. [Google Scholar] [CrossRef]
- Jones, F.R. A Review of Interphase Formation and Design in Fibre-Reinforced Composites. J. Adhes. Sci. Technol. 2010, 24, 171–202. [Google Scholar] [CrossRef]
- Sharma, M.; Gao, S.; Mäder, E.; Sharma, H.; Wei, L.Y.; Bijwe, J. Carbon fiber surfaces and composite interphases. Compos. Sci. Technol. 2014, 102, 35–50. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Mahmood, H.; Pegoretti, A. Recent advances in fiber/matrix interphase engineering for polymer composites. Prog. Mater. Sci. 2015, 73, 1–43. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Li, Q.; Shafei, S.; Huson, M.G.; Naebe, M. Carbon fibre surface modification using functionalized nanoclay: A hierarchical interphase for fibre-reinforced polymer composites. Compos. Sci. Technol. 2017, 148, 49–58. [Google Scholar] [CrossRef]
- Eyckens, D.J.; Stojcevski, F.; Hendlmeier, A.J.; Arnold, C.L.; Randall, J.D.; Perus, M.D.; Servinis, L.; Gengenbach, T.R.; Demir, B.; Walsh, T.R.; et al. An efficient high-throughput grafting procedure for enhancing carbon fiber-to-matrix interactions in composites. Chem. Eng. J. 2018, 353, 373–380. [Google Scholar] [CrossRef]
- Yao, X.; Gao, X.; Jiang, J.; Xu, C.; Deng, C.; Wang, J. Comparison of carbon nanotubes and graphene oxide coated carbon fiber for improving the interfacial properties of carbon fiber/epoxy composites. Compos. B 2018, 132, 170–177. [Google Scholar] [CrossRef]
- Hung, P.-Y.; Lau, K.-T.; Fox, B.; Hameed, N.; Lee, J.H.; Hui, D. Surface modification of carbon fibre using graphene–related materials for multifunctional composites. Compos. B 2018, 133, 240–257. [Google Scholar] [CrossRef]
- Randall, J.D.; Eyckens, D.J.; Servinis, L.; Stojcevski, F.; O′Dell, L.A.; Gengenbach, T.R.; Demir, B.; Walsh, T.R.; Henderson, L.C. Designing carbon fiber composite interfaces using a ‘graft-to’ approach: Surface grafting density versus interphase penetration. Carbon 2019, 146, 88–96. [Google Scholar] [CrossRef]
- Zhang, H.-P.; Han, W.; Tavakoli, J.; Zhang, Y.-P.; Lin, X.; Lu, X.; Ma, Y.; Tang, Y. Understanding interfacial interactions of polydopamine and glass fiber and their enhancement mechanisms in epoxy-based laminates. Compos. A. 2019, 116, 62–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Rhee, K.Y.; Hui, D.; Park, S.-J. A critical review of nanodiamond based nanocomposites: Synthesis, properties and applications. Compos. B 2018, 143, 19–27. [Google Scholar] [CrossRef]
- Chen, J.; Yan, L.; Song, W.; Xu, D. Interfacial characteristics of carbon nanotube-polymer composites: A review. Compos. A. 2018, 114, 149–169. [Google Scholar] [CrossRef]
- Pramanik, C.; Nepal, D.; Nathanson, M.; Gissinger, J.R.; Garley, A.; Berry, R.J.; Davijani, A.; Kumar, S.; Heinz, H. Molecular engineering of interphases in polymer/carbon nanotube composites to reach the limits of mechanical performance. Compos. Sci. Technol. 2018, 166, 86–94. [Google Scholar] [CrossRef]
- Cech, V.; Knob, A.; Hosein, H.-A.; Babik, A.; Lepcio, P.; Ondreas, F.; Drzal, L. Enhanced interfacial adhesion of glass fibers by tetravinylsilane plasma modification. Compos. A 2014, 58, 84–89. [Google Scholar] [CrossRef]
- Cech, V.; Knob, A.; Lasota, T.; Lukes, J.; Drzal, L.T. Surface modification of glass fibers by oxidized plasma coatings to improve interfacial shear strength in GF/polyester composites. Polym. Compos. 2019, 40, E186–E193. [Google Scholar] [CrossRef]
- Knob, A.; Lukes, J.; Drzal, L.T.; Cech, V. Further Progress in Functional Interlayers with Controlled Mechanical Properties Designed for Glass Fiber/Polyester Composites. Fibers 2018, 6, 58. [Google Scholar] [CrossRef]
- Friedrich, J.; Hidde, G. Ultra-Thin Plasma Polymer Films as a Novel Class of Adhesion Promoters: A Critical Review. Rev. Adhes. Adhes. 2015, 3, 1–52. [Google Scholar] [CrossRef]
- Bussey, D.; Perina, V.; Jones, F.R.; Cech, V. Effect of chemical modification on the mechanical properties of plasma-polymerized organosilicones. Prog. Org. Coatings 2018, 119, 85–90. [Google Scholar] [CrossRef]
- Cechalova, B.; Branecky, M.; Klapetek, P.; Cech, V. Optical Properties of Oxidized Plasma-Polymerized Organosilicones and Their Correlation with Mechanical and Chemical Parameters. Materials. 2019, 12, 539. [Google Scholar] [CrossRef]
- Cech, V.; Marek, A.; Knob, A.; Valter, J.; Branecky, M.; Plihal, P.; Vyskocil, J. Continuous surface modification of glass fibers in a roll-to-roll plasma-enhanced CVD reactor for glass fiber/polyester composites. Compos. Part A: Appl. Sci. Manuf. 2019, 121, 244–253. [Google Scholar] [CrossRef]
- Palesch, E.; Cech, V. Characterization of interlayer adhesion on single glass fibers and planar glass using the nanoscratch test technique. Thin Solid Films 2017, 636, 353–358. [Google Scholar] [CrossRef]
- ASTM D2344. Standard Test Method for Short-Beam Strength of Polymer Matrix Composite Materials and Their Laminates; American Society for Testing and Materials: West Conshohocken, PA, USA, 2001. [Google Scholar]
- Inagaki, N. Plasma Surface Modification and Plasma Polymerization; CRC Press: Boca Raton, FL, USA, 1996; ISBN 9781566763370. [Google Scholar]
- Palesch, E.; Knob, A.; Plichta, T.; Cech, V. Functional interlayers with controlled adhesion developed for polymer composites. Thin Solid Films 2018, 656, 37–43. [Google Scholar] [CrossRef]
- Bull, S.; Rickerby, D.; Matthews, A.; Leyland, A.; Pace, A.; Valli, J. The use of scratch adhesion testing for the determination of interfacial adhesion: The importance of frictional drag. Surf. Coatings Technol. 1988, 36, 503–517. [Google Scholar] [CrossRef]
- Bull, S.; Rickerby, D. New developments in the modelling of the hardness and scratch adhesion of thin films. Surf. Coatings Technol. 1990, 42, 149–164. [Google Scholar] [CrossRef]
- Lin-Vein, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Tolstoy, V.P.; Chernyshova, I.V.; Skryshevsky, V.A. Handbook of Infrared Spectroscopy of Ultrathin Films; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Cech, V.; Lichovnikova, S.; Sova, J.; Studynka, J. Surface free energy of silicon-based plasma polymer films. In Silanes and Other Coupling Agents, 1st ed.; Mittal, K.L., Ed.; VSP: Leiden, The Netherlands, 2009; Volume 5, pp. 333–348. ISBN 9789004165915. [Google Scholar]
- Drzal, L.T.; Herrera-Franco, P.J.; Ho, H. Fiber-matrix interface tests. In Comprehensive Composite Materials, 1st ed.; Kelly, A., Zweben, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Chapter 5.05; pp. 71–111. ISBN 0-080437230. [Google Scholar]
















© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zvonek, M.; Sirjovova, V.; Branecky, M.; Plichta, T.; Skacel, J.; Cech, V. Plasma Nanocoatings Developed to Control the Shear Strength of Polymer Composites. Polymers 2019, 11, 1188. https://doi.org/10.3390/polym11071188
Zvonek M, Sirjovova V, Branecky M, Plichta T, Skacel J, Cech V. Plasma Nanocoatings Developed to Control the Shear Strength of Polymer Composites. Polymers. 2019; 11(7):1188. https://doi.org/10.3390/polym11071188
Chicago/Turabian StyleZvonek, Milan, Veronika Sirjovova, Martin Branecky, Tomas Plichta, Josef Skacel, and Vladimir Cech. 2019. "Plasma Nanocoatings Developed to Control the Shear Strength of Polymer Composites" Polymers 11, no. 7: 1188. https://doi.org/10.3390/polym11071188
APA StyleZvonek, M., Sirjovova, V., Branecky, M., Plichta, T., Skacel, J., & Cech, V. (2019). Plasma Nanocoatings Developed to Control the Shear Strength of Polymer Composites. Polymers, 11(7), 1188. https://doi.org/10.3390/polym11071188