Solvent-Free Synthesis of Amidated Carboxymethyl Cellulose Derivatives: Effect on the Thermal Properties
Abstract
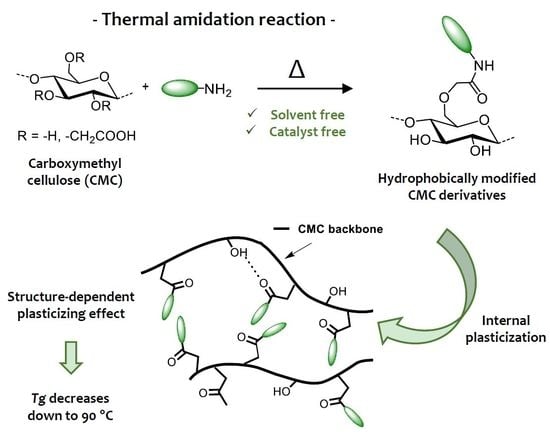
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Acidification of NaCMC
2.3. General Procedure for Thermal Amidation Reaction on HCMC
2.4. Characterizations
2.4.1. Size Exclusion Chromatography (SEC)
2.4.2. Infrared Analysis (IR)
2.4.3. Nuclear Magnetic Resonance (NMR)
2.4.4. Thermogravimetric Analysis (TGA)
2.4.5. Differential Scanning Calorimetry (DSC)
2.4.6. Dynamical Mechanical Analysis (DMA)
3. Results
3.1. Thermal Amidation of Carboxymethyl Cellulose
3.2. Thermal Properties of Amidated CMC Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Habibi, Y.; Lucia, L.A. Polysaccharide Building Blocks: A Sustainable Approach to the Development of Renewable Materials; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 9780470874196. [Google Scholar]
- van den Broek, L.A.M.; Knoop, R.J.I.; Kappen, F.H.J.; Boeriu, C.G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Kalia, S.; Avérous, L. Biodegradable and Biobased Polymers for Environmental and Biomedical Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; ISBN 9781119117360. [Google Scholar]
- Elschner, T.; Obst, F.; Heinze, T. Furfuryl- and Maleimido Polysaccharides: Synthetic Strategies Toward Functional Biomaterials. Macromol. Biosci. 2018, 18, 1800258. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Schmauder, H.-P.; Heinze, T. Cellulose. In Biopolymers Online; Vandamme, E.J., De Baets, S., Steinbüchel, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Dhuiège, B.; Pecastaings, G.; Sèbe, G. Sustainable Approach for the Direct Functionalization of Cellulose Nanocrystals Dispersed in Water by Transesterification of Vinyl Acetate. ACS Sustain. Chem. Eng. 2019, 7, 187–196. [Google Scholar] [CrossRef]
- Boulven, M.; Quintard, G.; Cottaz, A.; Joly, C.; Charlot, A.; Fleury, E. Homogeneous acylation of Cellulose diacetate: Towards bioplastics with tuneable thermal and water transport properties. Carbohydr. Polym. 2019, 206, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T.; Liebert, T. Unconventional methods in cellulose functionalization. Prog. Polym. Sci. 2001, 26, 1689–1762. [Google Scholar] [CrossRef]
- Jadhav, P. Global Carboxymethyl Cellulose Market 2019 by Manufacturers, Regions, Type and Application, Forecast to 2024; Global Info Research: Hong Kong, China, 2019. [Google Scholar]
- Pettignano, A.; Charlot, A.; Fleury, E. Carboxyl-functionalized derivatives of carboxymethyl cellulose: Towards advanced biomedical applications. Polym. Rev. 2019, 1–51. [Google Scholar] [CrossRef]
- Charpentier, D.; Mocanu, G.; Carpov, A.; Chapelle, S.; Merle, L.; Muller, G. New hydrophobically modified carboxymethylcellulose derivatives. Carbohydr. Polym. 1997, 33, 177–186. [Google Scholar] [CrossRef]
- Merle, L.; Charpentier, D.; Mocanu, G.; Chapelle, S. Comparison of the distribution pattern of associative carboxymethylcellulose derivatives. Eur. Polymer J. 1999, 35, 5–11. [Google Scholar] [CrossRef]
- Rosilio, V.; Albrecht, G.; Baszkin, A.; Merle, L. Surface properties of hydrophobically modified carboxymethylcellulose derivatives. Effect of salt and proteins. Colloids Surfaces B Biointerfaces 2000, 19, 163–172. [Google Scholar] [CrossRef]
- Sibikina, O.V.; Iozep, A.A.; Passet, B.V. Reactions of Carboxymethyl Polysaccharides and Their Ethyl Esters with Amines. Russ. J. Appl. Chem. 2004, 77, 263–266. [Google Scholar] [CrossRef]
- Ernsting, M.J.; Tang, W.L.; MacCallum, N.W.; Li, S.D. Preclinical pharmacokinetic, biodistribution, and anti-cancer efficacy studies of a docetaxel-carboxymethylcellulose nanoparticle in mouse models. Biomaterials 2012, 33, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Taubner, T.; Synytsya, A.; Čopíková, J. Preparation of amidated derivatives of carboxymethylcellulose. Int. J. Biol. Macromol. 2015, 72, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Movagharnezhad, N.; Moghadam, P.N. Folate-decorated carboxymethyl cellulose for controlled doxorubicin delivery. Colloid Polym. Sci. 2016, 294, 199–206. [Google Scholar] [CrossRef]
- Leggio, A.; Belsito, E.L.; De Luca, G.; Di Gioia, M.L.; Leotta, V.; Romio, E.; Siciliano, C.; Liguori, A. One-pot synthesis of amides from carboxylic acids activated using thionyl chloride. RSC Adv. 2016, 6, 34468–34475. [Google Scholar] [CrossRef]
- Magnani, A.; Rappuoli, R.; Lamponi, S.; Barbucci, R. Novel polysaccharide hydrogels: Characterization and properties. Polym. Adv. Technol. 2000, 11, 488–495. [Google Scholar] [CrossRef]
- Barbucci, R.; Leone, G.; Vecchiullo, A. Novel carboxymethylcellulose-based microporous hydrogels suitable for drug delivery. J. Biomater. Sci. Polym. Ed. 2004, 15, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Price, L.M.; Daly, W.H. Synthesis and characterization of a trifunctional aminoamide cellulose derivative. Biomacromolecules 2006, 7, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Charville, H.; Jackson, D.A.; Hodges, G.; Whiting, A.; Wilson, M.R. The Uncatalyzed Direct Amide Formation Reaction - Mechanism Studies and the Key Role of Carboxylic Acid H-Bonding. Eur. J. Org. Chem. 2011, 2011, 5981–5990. [Google Scholar] [CrossRef]
- Allen, C.L.; Chhatwal, A.R.; Williams, J.M.J. Direct amide formation from unactivated carboxylic acids and amines. Chem. Commun. 2012, 48, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Batelaan, J.G.; Van Der Horst, P.M. Method of Making Amide Modified Carboxyl-Containing Polysaccharide and Fatty Amide Modified Polysaccharide So Obtainable. US6103885A, 15 August 2000. [Google Scholar]
- Zabivalova, N.M.; Bochek, A.M.; Kalyuzhnaya, L.M.; Vlasova, E.N.; Volchek, B.Z. Carboxymethyl cellulose amides and their properties. Russ. J. Appl. Chem. 2003, 76, 1998–2002. [Google Scholar] [CrossRef]
- Wu, X.; Wang, M.; Xie, Y.; Chen, C.; Li, K.; Yuan, M.; Zhao, X.; Hou, Z. Carboxymethyl cellulose supported ionic liquid as a heterogeneous catalyst for the cycloaddition of CO2 to cyclic carbonate. Appl. Catal. A Gen. 2016, 519, 146–154. [Google Scholar] [CrossRef]
- Chocholoušová, J.; Vacek, J.; Hobza, P. Acetic Acid Dimer in the Gas Phase, Nonpolar Solvent, Microhydrated Environment, and Dilute and Concentrated Acetic Acid: Ab Initio Quantum Chemical and Molecular Dynamics Simulations. J. Phys. Chem. A 2003, 107, 3086–3092. [Google Scholar] [CrossRef]
- Arnold, K.; Batsanov, A.S.; Davies, B.; Whiting, A. Synthesis, evaluation and application of novel bifunctional N,N-di-isopropylbenzylamineboronic acid catalysts for direct amide formation between carboxylic acids and amines. Green Chem. 2008, 10, 124–134. [Google Scholar] [CrossRef]
- Cossy, J.; Pale-Grosdemange, C. A convenient synthesis of amides from carboxylic acids and primary amines. Tetrahedron Lett. 1989, 30, 2771–2774. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T.; Koschella, A. Esterification of Polysaccharides; Springer-Verlag: Berlin/Heidelberg, Germany, 2006; ISBN 3540321128. [Google Scholar]
- Kono, H.; Oshima, K.; Hashimoto, H.; Shimizu, Y.; Tajima, K. NMR characterization of sodium carboxymethyl cellulose: Substituent distribution and mole fraction of monomers in the polymer chains. Carbohydr. Polym. 2016, 146, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Oshima, K.; Hashimoto, H.; Shimizu, Y.; Tajima, K. NMR characterization of sodium carboxymethyl cellulose 2: Chemical shift assignment and conformation analysis of substituent groups. Carbohydr. Polym. 2016, 150, 241–249. [Google Scholar] [CrossRef]
- Biallas, P.; Häring, A.P.; Kirsch, S.F. Cleavage of 1,3-dicarbonyls through oxidative amidation. Org. Biomol. Chem. 2017, 15, 3184–3187. [Google Scholar] [CrossRef]
- Wan, H.; Holmén, A.; Någård, M.; Lindberg, W. Rapid screening of pKa values of pharmaceuticals by pressure-assisted capillary electrophoresis combined with short-end injection. J. Chromatogr. A 2002, 979, 369–377. [Google Scholar] [CrossRef]
- Matulis, D.; Bloomfield, V.A. Thermodynamics of the hydrophobic effect. I. Coupling of aggregation and pK(a) shifts in solutions of aliphatic amines. Biophys. Chem. 2001, 93, 37–51. [Google Scholar] [CrossRef]
- Biswal, D.R.; Singh, R.P. Characterisation of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbohydr. Polym. 2004, 57, 379–387. [Google Scholar] [CrossRef]
- Uskoković, V. Composites comprising cholesterol and carboxymethyl cellulose. Colloids Surf. B Biointerfaces 2008, 61, 250–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Huang, Z.; Yuan, X.; Wang, X.; Li, M. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr. Polym. 2010, 79, 145–153. [Google Scholar] [CrossRef]
- Lin, X.; Li, Y.; Chen, Z.; Zhang, C.; Luo, X.; Du, X.; Huang, Y. Synthesis, characterization and electrospinning of new thermoplastic carboxymethyl cellulose (TCMC). Chem. Eng. J. 2013, 215–216, 709–720. [Google Scholar] [CrossRef]
- Ramiah, M.V. Thermogravimetric and differential thermal analysis of cellulose, hemicellulose, and lignin. J. Appl. Polym. Sci. 1970, 14, 1323–1337. [Google Scholar] [CrossRef]
- Moldoveanu, S.C.; David, V. Chemical degradation of polymers and pyrolysis. J. Chromatogr. Libr. 2002, 65, 847–917. [Google Scholar]
- Chen, Z.; Zhang, J.; Xiao, P.; Tian, W.; Zhang, J. Novel Thermoplastic Cellulose Esters Containing Bulky Moieties and Soft Segments. ACS Sustain. Chem. Eng. 2018, 6, 4931–4939. [Google Scholar] [CrossRef]
- Vaca-Garcia, C.; Gozzelino, G.; Glasser, W.G.; Borredon, M.E. Dynamic mechanical thermal analysis transitions of partially and fully substituted cellulose fatty esters. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 281–288. [Google Scholar] [CrossRef]
- El-sayed, S.; Mahmoud, K.H.; Fatah, A.A.; Hassen, A. DSC, TGA and dielectric properties of carboxymethyl cellulose / polyvinyl alcohol blends. Phys. B Phys. Condens. Matter 2011, 406, 4068–4076. [Google Scholar] [CrossRef]
- Mura, P.; Gratteri, P.; Faucci, M.T. Compatibility Studies of Multicomponent Tablet Formulations. DSC and experimental mixture design. J. Therm. Anal. Calorim. 2002, 68, 541–551. [Google Scholar] [CrossRef]
- Siqueira, E.J.; Salon, M.B.; Mauret, E. The effects of sodium chloride (NaCl) and residues of cellulosic fibres derived from sodium carboxymethylcellulose (NaCMC) synthesis on thermal and mechanical properties of CMC films. Ind. Crop. Prod. 2015, 72, 87–96. [Google Scholar] [CrossRef]












| Entry | Sample | R’ | DSCM | Mass Yield (%) | DSamide 1 | DSsub 1 | Substitution Efficiency (%) 2 | Tdonset (°C) 3 | Tdmax (°C) 4 | Tg (°C) 5 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NaCMC | - | 1.2 | - | - | - | - | 225 | 288 | ND6 |
| 2 | HCMC | - | 1.2 | 86 | - | - | - | ND6 | 319 | ND6 |
| 3 | CMC1.2-Benz |  | 1.2 | 75 | 0.95 | 1 | 83 | 190 | 323 | 129 |
| 4 | CMC0.9-Benz |  | 0.9 | 68 | 0.6 | 0.7 | 78 | 185 | 312 | 138 |
| 5 | CMC0.7-Benz |  | 0.7 | 66 | 0.5 | 0.6 | 85 | 190 | 309 | 140 |
| 6 | CMC-PE |  | 1.2 | 76 | 0.5 | 0.85 | 71 | 190 | 312 | 122 |
| 7 | CMC-PP |  | 1.2 | 70 | 0.55 | 0.9 | 75 | 180 | 313 | 105 |
| 8 | CMC-PB |  | 1.2 | 57 | 0.5 | 0.8 | 67 | 185 | 319 | 90 |
| 9 | CMC-Und |  | 1.2 | 69 | 0.7 | 0.8 | 67 | 140 | 334 | ND6 |
| 10 | CMC-Dod |  | 1.2 | 67 | 0.65 | 0.8 | 67 | 150 | 332 | ND6 |
| 11 | CMC-Furf |  | 1.2 | 68 | 0.75 | 0.95 | 79 | 180 | 318 | 147 |
| 12 | CMC-Thio |  | 1.2 | 76 | 0.7 | 0.85 | 71 | 130 | 318 | 115 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pettignano, A.; Charlot, A.; Fleury, E. Solvent-Free Synthesis of Amidated Carboxymethyl Cellulose Derivatives: Effect on the Thermal Properties. Polymers 2019, 11, 1227. https://doi.org/10.3390/polym11071227
Pettignano A, Charlot A, Fleury E. Solvent-Free Synthesis of Amidated Carboxymethyl Cellulose Derivatives: Effect on the Thermal Properties. Polymers. 2019; 11(7):1227. https://doi.org/10.3390/polym11071227
Chicago/Turabian StylePettignano, Asja, Aurélia Charlot, and Etienne Fleury. 2019. "Solvent-Free Synthesis of Amidated Carboxymethyl Cellulose Derivatives: Effect on the Thermal Properties" Polymers 11, no. 7: 1227. https://doi.org/10.3390/polym11071227
APA StylePettignano, A., Charlot, A., & Fleury, E. (2019). Solvent-Free Synthesis of Amidated Carboxymethyl Cellulose Derivatives: Effect on the Thermal Properties. Polymers, 11(7), 1227. https://doi.org/10.3390/polym11071227