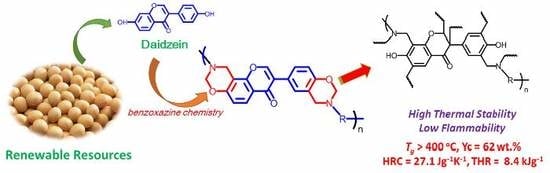
Synthesis of Highly Thermally Stable Daidzein-Based Main-Chain-Type Benzoxazine Resins
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
2.3. Synthesis of the Main-Chain-Type Benzoxazine Starting from Daidzein, 4,4′-Diaminodiphenylmethane and Paraformaldehyde (Abbreviated as Poly(Dd-Ddm)Main)
2.4. Synthesis of the Main-Chain-Type Benzoxazine Starting from Daidzein, 1,6-Hexamethylenediamine and Paraformaldehyde (Abbreviated as Poly(Dd-Hda)Main)
2.5. Polymerization of Daidzein-Based Main-Chain-Type Benzoxazines
3. Result and Discussion
3.1. Synthesis of Daidzein-Based Main-Chain-Type Benzoxazines
3.2. Thermally Activated Polymerization Behaviors
3.3. Thermal and Heat Release Properties of Polybenzoxazines
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ning, X.; Ishida, H. Phenolic materials via ring-opening polymerization: Synthesis and characterization of bisphenol-A based benzoxazines and their polymers. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 1121–1129. [Google Scholar] [CrossRef]
- Ghosh, N.N.; Kiskan, B.; Yagci, Y. Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties. Prog. Polym. Sci. 2007, 32, 1344–1391. [Google Scholar] [CrossRef]
- Ishida, H.; Agag, T. Handbook of Benzoxazine Resins; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Ishida, H.; Froimowicz, P. Advanced and Emerging Polybenzoxazine Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Ishida, H.; Low, H.Y. A study on the volumetric expansion of benzoxazine-based phenolic resin. Macromolecules 1997, 30, 1099–1106. [Google Scholar] [CrossRef]
- Zhang, K.; Qiu, J.; Li, S.; Shang, Z.; Wang, J. Remarkable improvement of thermal stability of main-chain benzoxazine oligomer by incorporation o-norbornene as terminal functionality. J. Appl. Polym. Sci. 2017, 134, 45408. [Google Scholar] [CrossRef]
- Demir, K.D.; Kiskan, B.; Yagci, Y. Thermally curable acetylene-containing main-chain benzoxazine polymers via sonogashira coupling reaction. Macromolecules 2011, 44, 1801–1807. [Google Scholar] [CrossRef]
- Zhang, K.; Tan, X.X.; Wang, Y.T.; Ishida, H. Unique self-catalyzed cationic ring-opening polymerization of a high performance deoxybenzoin-based 1,3-benzoxazine monomer. Polymer 2019, 168, 8–15. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, X. Catalyst-free and low-temperature terpolymerization in a single-component benzoxazine resin containing both norbornene and acetylene functionalities. Macromolecules 2018, 51, 6524–6533. [Google Scholar] [CrossRef]
- Wang, C.F.; Su, Y.C.; Kuo, S.W. Low-surface-free-energy materials based on polybenzoxazines. Angew. Chem. Int. Ed. 2010, 45, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Li, H.T.; Huang, S.C.; Chen, W.B.; Sun, K.W.; Chang, F.C. Synthesis and performance enhancement of novel novel polybenzoxazine with low surface free energy. Polym. Int. 2011, 60, 1089–1096. [Google Scholar] [CrossRef]
- Arslan, M.; Motallebzadeh, A.; Kiskan, B.; Demirel, A.L.; Kumbaraci, I.V.; Yagci, Y. Combining benzoxazine and ketene chemistries for self-healing of high performance thermoset surfaces. Polym. Chem. 2018, 9, 2031–2039. [Google Scholar] [CrossRef]
- Wu, J.; Xi, Y.; Mccandless, G.T.; Xie, Y.; Menon, R.; Patel, Y.; Menon, R.; Patel, Y.; Yang, D.J.; Iacono, S.T.; et al. Synthesis and characterization of partially fluorinated polybenzoxazine resins utilizing octafluorocyclopentene as a versatile building block. Macromolecules 2015, 48, 6087–6095. [Google Scholar] [CrossRef]
- Zhang, K.; Han, L.; Froimowicz, P.; Ishida, H. A smart latent catalyst containing o-trifluoroacetamide functional benzoxazine: Precursor for low temperature formation of very high Performance polybenzoxazole with low dielectric constant and high thermal stability. Macromolecules 2017, 50, 6552–6560. [Google Scholar] [CrossRef]
- Zhang, K.; Shang, Z.; Evans, C.J.; Han, L.; Ishida, H.; Yang, S. Benzoxazine atropisomers: Intrinsic atropisomerization mechanism and conversion to high performance thermosets. Macromolecules 2018, 51, 7574–7585. [Google Scholar] [CrossRef]
- Zhang, K.; Han, M.C.; Han, L.; Ishida, H. Resveratrol-based tri-functional benzoxazines: Synthesis, characterization, polymerization, and thermal and flame retardant properties. Eur. Polym. J. 2019, 116, 526–533. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.Q.; Shang, Z.K.; Evans, C.J.; Yang, S.F. Effect of end-caps on the atropisomerization, polymerization and thermal properties of ortho-imide functional benzoxazines. Polymers 2019, 11, 399. [Google Scholar] [CrossRef]
- Chernykh, A.; Liu, J.; Ishida, H. Synthesis and properties of a new crosslinkable polymer containing benzoxazine moiety in the main chain. Polymer 2006, 47, 7664–7669. [Google Scholar] [CrossRef]
- Demir, K.D.; Kiskan, B.; Aydogan, B.; Yagci, Y. Thermally curable main-chain benzoxazine prepolymers via polycondensation route. React. Funct. Polym. 2013, 73, 346–359. [Google Scholar] [CrossRef]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B. Biobased thermosetting epoxy: Present and future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef]
- Liggins, J.; Bluck, L.J.; Runswick, S.; Atkinson, C.; Coward, W.A.; Bingham, S.A. Daidzein and genistein content of fruits and nuts. J. Nutr. Biochem. 2000, 11, 326–331. [Google Scholar] [CrossRef]
- Ye, H.; Dudley, S.Z.; Shaw, I.C. Escherichia coli biotransformation of daidzein fermentation products from soy-based foods-relevance to food oestrogenicity-based functionality. Int. J. Food Sci. Technol. 2017, 52, 1082–1091. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Coward, L.; Barnes, N.C.; Setchell, K.D.; Barnes, S. Genistein, daidzein, and their beta.-glycoside conjugates: Antitumor isoflavones in soybean foods from American and Asian diets. J. Agric. Food Chem. 1993, 41, 1961–1967. [Google Scholar] [CrossRef]
- Dai, J.; Peng, Y.; Teng, N.; Liu, Y.; Liu, C.; Shen, X.; Mahmud, S.; Zhu, J.; Liu, X. High-performing and fire-resistant biobased epoxy resin from renewable sources. ACS. Sustain. Chem. Eng. 2018, 6, 7589–7599. [Google Scholar] [CrossRef]
- Dai, J.; Teng, N.; Peng, Y.; Liu, Y.; Cao, L.; Zhu, J.; Liu, X. Biobased Benzoxazine Derived from Daidzein and Furfurylamine: Microwave-Assisted Synthesis and Thermal Properties Investigation. ChemSusChem 2018, 11, 3175–3183. [Google Scholar] [CrossRef]
- Han, L.; Salum, M.L.; Zhang, K.; Froimowicz, P.; Ishida, H. Intrinsic Self-Initiating Thermal Ring-Opening Polymerization of 1,3-Benzoxazines Without the Influence of Impurities Using Very High Purity Crystals. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3434–3445. [Google Scholar] [CrossRef]
- Dunkers, J.; Ishida, H. Vibrational assignments of 3-alkyl-3, 4-dihydro-6-methyl-2H-1,3-benzoxazines in the Fingerprint Region. Spectrochim. Acta Part A 1995, 51, 1061–1074. [Google Scholar] [CrossRef]
- Han, L.; Iguchi, D.; Gil, P.; Heyl, T.R.; Sedwick, V.M.; Arza, C.R.; Ohashi, S.; Lacks, D.J.; Ishida, H. Oxazine ring-related vibrational modes of benzoxazine monomers using fully aromatically substituted, deuterated, 15N isotope exchanged, and oxazine-ring-substituted compounds and theoretical calculations. J. Phys. Chem. A 2017, 121, 6269–6282. [Google Scholar] [CrossRef]
- Takeichi, T.; Kano, T.; Agag, T. Synthesis and thermal cure of high molecular weight polybenzoxazine precursors and the properties of the thermosets. Polymer 2005, 46, 12172–12180. [Google Scholar] [CrossRef]
- Zeng, M.; Chen, J.; Xu, Q.; Huang, Y.; Feng, Z.; Gu, Y. A facile method for the preparation of aliphatic main-chain benzoxazine copolymers with high-frequency low dielectric constants. Polym. Chem. 2018, 9, 2913–2925. [Google Scholar] [CrossRef]
- Baqar, M.; Agag, T.; Ishida, H.; Qutubuddin, S. Poly(benzoxazine-co-urethane)s: A new concept for phenolic/urethane copolymers via one-pot method. Polymer 2011, 52, 307–317. [Google Scholar] [CrossRef]
- Lyon, R.E.; Safronava, N.; Quintiere, J.G.; Stoliarov, S.I.; Walters, R.N.; Crowley, S. Materials properties and fir test results. Fire Mater. 2014, 38, 264–278. [Google Scholar] [CrossRef]
- Agag, T.; Liu, J.; Graf, R.; Spiess, H.W.; Ishida, H. Benzoxazole resin: A novel class of thermoset polymer via smart benzoxazine resin. Macromolecules 2012, 45, 8991–8997. [Google Scholar] [CrossRef]
- Walters, R.N.; Lyon, R.E. Molar group contributions to polymer flammability. J. Appl. Polym. Sci. 2003, 87, 548–563. [Google Scholar] [CrossRef]











| Benzoxazine | Mn (Daltons) | Mw (Daltons) | Dispersity Index |
|---|---|---|---|
| poly(Dd-ddm)main | 7019 | 9781 | 1.39 |
| poly(Dd-hda)main | 5754 | 8281 | 1.44 |
| Sample | Tg (°C) | N2 | Air | HRC (J g−1K−1) | THR (kJ g−1) | |||
|---|---|---|---|---|---|---|---|---|
| Td5 (°C) | Td10 (°C) | Yc | Td5 (°C) | Td10 (°C) | ||||
| poly(Dd-hda)Xmain | 331 | 346 | 383 | 53 | 334 | 375 | 83.2 | 14.3 |
| poly(Dd-ddm)Xmain | >400 | 383 | 426 | 62 | 373 | 401 | 27.1 | 8.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; You, S.; Wang, Y.; Zhang, K.; Yang, S. Synthesis of Highly Thermally Stable Daidzein-Based Main-Chain-Type Benzoxazine Resins. Polymers 2019, 11, 1341. https://doi.org/10.3390/polym11081341
Han M, You S, Wang Y, Zhang K, Yang S. Synthesis of Highly Thermally Stable Daidzein-Based Main-Chain-Type Benzoxazine Resins. Polymers. 2019; 11(8):1341. https://doi.org/10.3390/polym11081341
Chicago/Turabian StyleHan, Mengchao, Sijia You, Yuting Wang, Kan Zhang, and Shengfu Yang. 2019. "Synthesis of Highly Thermally Stable Daidzein-Based Main-Chain-Type Benzoxazine Resins" Polymers 11, no. 8: 1341. https://doi.org/10.3390/polym11081341
APA StyleHan, M., You, S., Wang, Y., Zhang, K., & Yang, S. (2019). Synthesis of Highly Thermally Stable Daidzein-Based Main-Chain-Type Benzoxazine Resins. Polymers, 11(8), 1341. https://doi.org/10.3390/polym11081341