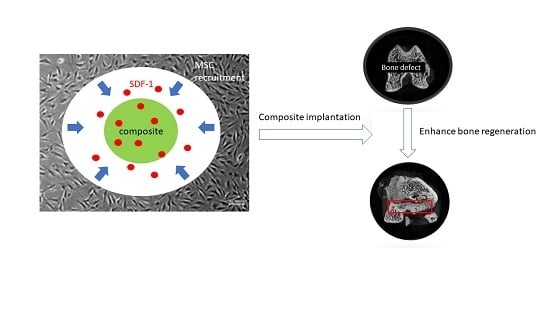
The Development of Gelatin/Hyaluronate Copolymer Mixed with Calcium Sulfate, Hydroxyapatite, and Stromal-Cell-Derived Factor-1 for Bone Regeneration Enhancement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hydroxyapatite
2.3. Preparation of Gel-HA/CS/HAP Composite with SDF-1
2.4. Material Characterization
2.4.1. X-ray Diffraction (XRD) Analysis
2.4.2. Fourier-Transform Infrared Spectroscopy Analysis
2.4.3. Swelling Ratio
2.4.4. Degradation Test
2.4.5. SDF-1 Release Profile
2.5. In Vitro Study
2.5.1. Cell Culture
2.5.2. Cell Viability
2.5.3. MSC Recruitment Test
2.6. In Vivo Study
2.6.1. Implantation of Composite in Rat Femur Bone Defect Model
2.6.2. Blood Analysis
2.6.3. Two-Photon Excitation Microscopy
2.6.4. Micro-CT
2.6.5. Histological Analysis
3. Results and Discussion
3.1. XRD Analysis
3.2. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
3.3. Swelling Ratio
3.4. Degradation Rate
3.5. SDF-1 Release Profile
3.6. Cell Viability
3.7. MSC Recruitment Test
3.8. In Vivo Study
3.8.1. Blood Analysis
3.8.2. Two-Photon Excitation Microscopy
3.8.3. Micro-CT
3.8.4. Histological Analysis
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Nauth, A.; McKee, M.D.; Einhorn, T.A.; Watson, J.T.; Li, R.; Schemitsch, E.H. Managing bone defects. J. Orthop. Trauma 2011, 25, 462–466. [Google Scholar] [CrossRef]
- Fillingham, Y.; Jacobs, J. Bone grafts and their substitutes. Bone Jt. J. 2016, 98-B, 6–9. [Google Scholar] [CrossRef]
- Lobb, D.C.; DeGeorge, B.R., Jr.; Chhabra, A.B. Bone graft substitutes: Current concepts and future expectations. J. Hand Surg. Am. 2019, 44, 497–505.e492. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Dressman, H. Uber knochenplombierung. Beitr Klin Chir 1892, 9, 804–810. [Google Scholar]
- Kelly, C.M.; Wilkins, R.M.; Gitelis, S.; Hartjen, C.; Watson, J.T.; Kim, P.T. The use of a surgical grade calcium sulfate as a bone graft substitute: Results of a multicenter trial. Clin. Orthop. Relat. Res. 2001, 382, 42–50. [Google Scholar] [CrossRef]
- Pietrzak, W.S.; Ronk, R. Calcium sulfate bone void filler: A review and a look ahead. J. Craniofac. Surg. 2000, 11, 327–333. [Google Scholar] [CrossRef]
- Jepegnanam, T.S.; von Schroeder, H.P. Rapid resorption of calcium sulfate and hardware failure following corrective radius osteotomy: 2 case reports. J. Hand Surg. Am. 2012, 37, 477–480. [Google Scholar] [CrossRef]
- Lee, G.H.; Khoury, J.G.; Bell, J.E.; Buckwalter, J.A. Adverse reactions to osteoset bone graft substitute, the incidence in a consecutive series. Iowa Orthop. J. 2002, 22, 35–38. [Google Scholar]
- Hing, K.A.; Wilson, L.F.; Buckland, T. Comparative performance of three ceramic bone graft substitutes. Spine J. 2007, 7, 475–490. [Google Scholar] [CrossRef]
- Holmes, R.E. Bone regeneration within a coralline hydroxyapatite implant. Plast. Reconstr. Surg. 1979, 63, 626–633. [Google Scholar] [CrossRef]
- Even, J.; Eskander, M.; Kang, J. Bone morphogenetic protein in spine surgery: Current and future uses. J. Am. Acad. Orthop. Surg. 2012, 20, 547–552. [Google Scholar]
- Gruskin, E.; Doll, B.A.; Futrell, F.W.; Schmitz, J.P.; Hollinger, J.O. Demineralized bone matrix in bone repair: History and use. Adv. Drug Deliv. Rev. 2012, 64, 1063–1077. [Google Scholar] [CrossRef]
- Shehadi, J.A.; Elzein, S.M. Review of commercially available demineralized bone matrix products for spinal fusions: A selection paradigm. Surg. Neurol. Int. 2017, 8, 203. [Google Scholar] [CrossRef] [Green Version]
- Boyce, T.; Edwards, J.; Scarborough, N. Allograft bone. The influence of processing on safety and performance. Orthop. Clin. N. Am. 1999, 30, 571–581. [Google Scholar] [CrossRef]
- Finkemeier, C.G. Bone-grafting and bone-graft substitutes. J. Bone Jt. Surg. Am. 2002, 84, 454–464. [Google Scholar] [CrossRef]
- Kitaori, T.; Ito, H.; Schwarz, E.M.; Tsutsumi, R.; Yoshitomi, H.; Oishi, S.; Nakano, M.; Fujii, N.; Nagasawa, T.; Nakamura, T. Stromal cell-derived factor 1/cxcr4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2009, 60, 813–823. [Google Scholar] [CrossRef]
- Otsuru, S.; Tamai, K.; Yamazaki, T.; Yoshikawa, H.; Kaneda, Y. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the cxcr4/stromal cell-derived factor-1 pathway. Stem Cells 2008, 26, 223–234. [Google Scholar] [CrossRef]
- Segers, V.F.M.; Tokunou, T.; Higgins, L.J.; MacGillivray, C.; Gannon, J.; Lee, R.T. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation 2007, 116, 1683–1692. [Google Scholar] [CrossRef]
- Knight, M.N.; Hankenson, K.D. Mesenchymal stem cells in bone regeneration. Adv. Wound Care 2013, 2, 306–316. [Google Scholar] [CrossRef]
- Jin, Y.Z.; Lee, J.H. Mesenchymal stem cell therapy for bone regeneration. Clin. Orthop. Surg. 2018, 10, 271–278. [Google Scholar] [CrossRef]
- Kang, H.W.; Tabata, Y.; Ikada, Y. Fabrication of porous gelatin scaffolds for tissue engineering. Biomaterials 1999, 20, 1339–1344. [Google Scholar] [CrossRef]
- Chen, W.Y.J.; Abatangelo, G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999, 7, 79–89. [Google Scholar] [CrossRef]
- Akao, M.; Aoki, H.; Kato, K. Mechanical-properties of sintered hydroxyapatite for prosthetic applications. J. Mater. Sci. 1981, 16, 809–812. [Google Scholar] [CrossRef]
- Shyong, Y.J.; Wang, M.H.; Kuo, L.W.; Su, C.F.; Kuo, W.T.; Chang, K.C.; Lin, F.H. Mesoporous hydroxyapatite as a carrier of olanzapine for long-acting antidepression treatment in rats with induced depression. J. Control. Release 2017, 255, 62–72. [Google Scholar] [CrossRef]
- Hung, C.J.; Yao, C.L.; Cheng, F.C.; Wu, M.L.; Wang, T.H.; Hwang, S.M. Establishment of immortalized mesenchymal stromal cells with red fluorescence protein expression for in vivo transplantation and tracing in the rat model with traumatic brain injury. Cytotherapy 2010, 12, 455–465. [Google Scholar] [CrossRef]
- Liu, M.; Lv, Y. Reconstructing bone with natural bone graft: A review of in vivo studies in bone defect animal model. Nanomaterials 2018, 8, 999. [Google Scholar] [CrossRef]
- Hollinger, J.O.; Kleinschmidt, J.C. The critical size defect as an experimental model to test bone repair materials. J. Craniofac. Surg. 1990, 1, 60–68. [Google Scholar] [CrossRef]
- Kondo, N.; Ogose, A.; Tokunaga, K.; Ito, T.; Arai, K.; Kudo, N.; Inoue, H.; Irie, H.; Endo, N. Bone formation and resorption of highly purified beta-tricalcium phosphate in the rat femoral condyle. Biomaterials 2005, 26, 5600–5608. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.-K.; Li, L.; Qin, L.; Wang, X.-L.; Lai, Y.-X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, J.P.; Hollinger, J.O. The critical size defect as an experimental-model for craniomandibulofacial nonunions. Clin. Orthop. Relat. R. 1986, 205, 299–308. [Google Scholar] [CrossRef]
- Sun, J.; Mou, C.; Shi, Q.; Chen, B.; Hou, X.; Zhang, W.; Li, X.; Zhuang, Y.; Shi, J.; Chen, Y.; et al. Controlled release of collagen-binding sdf-1alpha from the collagen scaffold promoted tendon regeneration in a rat achilles tendon defect model. Biomaterials 2018, 162, 22–33. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, Y.; Li, Q.; Chen, B.; Hou, X.; Xiao, Z.; Dai, J. Controlled release of collagen-binding sdf-1alpha improves cardiac function after myocardial infarction by recruiting endogenous stem cells. Sci. Rep. 2016, 6, 26683. [Google Scholar] [CrossRef]
- Wang, W.; Li, W.; Ong, L.L.; Furlani, D.; Kaminski, A.; Liebold, A.; Lutzow, K.; Lendlein, A.; Wang, J.; Li, R.K.; et al. Localized sdf-1alpha gene release mediated by collagen substrate induces cd117 stem cells homing. J. Cell. Mol. Med. 2010, 14, 392–402. [Google Scholar] [CrossRef]
- Ishii, M.; Fujimori, S.; Kaneko, T.; Kikuta, J. Dynamic live imaging of bone: Opening a new era with ‘bone histodynametry’. J. Bone Miner. Metab. 2013, 31, 507–511. [Google Scholar] [CrossRef]
- Sano, H.; Kikuta, J.; Furuya, M.; Kondo, N.; Endo, N.; Ishii, M. Intravital bone imaging by two-photon excitation microscopy to identify osteocytic osteolysis in vivo. Bone 2015, 74, 134–139. [Google Scholar] [CrossRef]
- Campagnola, P.J.; Loew, L.M. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat. Biotechnol. 2003, 21, 1356–1360. [Google Scholar] [CrossRef]
- Faruq, O.; Kim, B.; Padalhin, A.R.; Lee, G.H.; Lee, B.T. A hybrid composite system of biphasic calcium phosphate granules loaded with hyaluronic acid-gelatin hydrogel for bone regeneration. J. Biomater. Appl. 2017, 32, 433–445. [Google Scholar] [CrossRef]
- Subramaniam, S.; Fang, Y.-H.; Sivasubramanian, S.; Lin, F.-H.; Lin, C.-P. Hydroxyapatite-calcium sulfate-hyaluronic acid composite encapsulated with collagenase as bone substitute for alveolar bone regeneration. Biomaterials 2016, 74, 99–108. [Google Scholar] [CrossRef]
- Teotia, A.K.; Gupta, A.; Raina, D.B.; Lidgren, L.; Kumar, A. Gelatin-modified bone substitute with bioactive molecules enhance cellular interactions and bone regeneration. ACS Appl. Mater. Interfaces 2016, 8, 10775–10787. [Google Scholar] [CrossRef]
- Lin, W.P.; Xu, L.L.; Zwingenberger, S.; Gibon, E.; Goodman, S.B.; Li, G. Mesenchymal stem cells homing to improve bone healing. J. Orthop. Transl. 2017, 9, 19–27. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Gu, Y.; Xu, Y.; Liu, Y.; Li, B.; Chen, L. Sequential and sustained release of sdf-1 and bmp-2 from silk fibroin-nanohydroxyapatite scaffold for the enhancement of bone regeneration. Biomaterials 2016, 106, 205–216. [Google Scholar] [CrossRef]
- Lapidot, T. Mechanism of human stem cell migration and repopulation of nod/scid and b2mnull nod/scid mice. The role of sdf-1/cxcr4 interactions. Ann. N. Y. Acad. Sci. 2001, 938, 83–95. [Google Scholar] [CrossRef]












| Control | Without SDF-1 | With SDF-1 | Reference * | |
|---|---|---|---|---|
| ALKP (U/L) | 310.67 | 276 | 253 | 39–216 |
| Ca (mg/dL) | 11.63 | 12.1 | 12.27 | 8–15 |
| LDH (U/L) | 656.33 | 459.33 | 430 | 300–700 |
| Control | Without SDF-1 | With SDF-1 | Reference * | |
|---|---|---|---|---|
| ALKP (U/L) | 260.33 | 214.67 | 214.67 | 39–216 |
| Ca (mg/dL) | 10.97 | 10.77 | 10.83 | 8–15 |
| LDH (U/L) | 586 | 368.67 | 771 | 300–700 |
| Control | Without SDF-1 | With SDF-1 | Reference * | |
|---|---|---|---|---|
| RBC (M/µL) | 8.32 | 8.45 | 8.3 | 7.37–9.25 |
| HGB (g/dL) | 14.73 | 15.3 | 15.1 | 14.4–17.6 |
| HCT (%) | 44.4 | 46.37 | 45.63 | 36–46 |
| MCV (fL) | 53.37 | 54.83 | 54.97 | 47–52 |
| MCH (pg) | 17.7 | 18.1 | 18.2 | 17–21 |
| MCHC (g/dL) | 33.2 | 33 | 33.1 | 35–43 |
| WBC (K/µL) | 11.52 | 13.99 | 12.33 | 6.19–12.55 |
| NEUT (%) | 16.67 | 14.13 | 19.23 | 1–29 |
| LYMPH (%) | 75.27 | 80.9 | 73.57 | 70–99 |
| MONO (%) | 5.23 | 2.97 | 5.13 | 0–6 |
| EO (%) | 2.63 | 1.9 | 2 | 0–3 |
| BASO (%) | 0.2 | 0.1 | 0.07 | 0–2 |
| Control | Without SDF-1 | With SDF-1 | Reference * | |
|---|---|---|---|---|
| RBC (M/µL) | 9.16 | 9.18 | 8.87 | 7.37–9.25 |
| HGB (g/dL) | 15.47 | 15.4 | 15.00 | 14.4–17.6 |
| HCT (%) | 45 | 45.47 | 43.75 | 36–46 |
| MCV (fL) | 49.14 | 49.58 | 49.35 | 47–52 |
| MCH (pg) | 16.90 | 16.79 | 16.92 | 17–21 |
| MCHC (g/dL) | 34.37 | 33.89 | 34.29 | 35–43 |
| WBC (K/µL) | 10.29 | 10.33 | 10.05 | 6.19–12.55 |
| NEUT (%) | 16.67 | 14.13 | 19.23 | 1–29 |
| LYMPH (%) | 75.27 | 80.9 | 73.57 | 70–99 |
| MONO (%) | 5.23 | 2.97 | 5.13 | 0–6 |
| EO (%) | 2.63 | 1.9 | 2 | 0–3 |
| BASO (%) | 0.2 | 0.1 | 0.07 | 0–2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-L.; Hsieh, C.-Y.; Yeh, C.-Y.; Lin, F.-H. The Development of Gelatin/Hyaluronate Copolymer Mixed with Calcium Sulfate, Hydroxyapatite, and Stromal-Cell-Derived Factor-1 for Bone Regeneration Enhancement. Polymers 2019, 11, 1454. https://doi.org/10.3390/polym11091454
Chang Y-L, Hsieh C-Y, Yeh C-Y, Lin F-H. The Development of Gelatin/Hyaluronate Copolymer Mixed with Calcium Sulfate, Hydroxyapatite, and Stromal-Cell-Derived Factor-1 for Bone Regeneration Enhancement. Polymers. 2019; 11(9):1454. https://doi.org/10.3390/polym11091454
Chicago/Turabian StyleChang, Yun-Liang, Chia-Ying Hsieh, Chao-Yuan Yeh, and Feng-Huei Lin. 2019. "The Development of Gelatin/Hyaluronate Copolymer Mixed with Calcium Sulfate, Hydroxyapatite, and Stromal-Cell-Derived Factor-1 for Bone Regeneration Enhancement" Polymers 11, no. 9: 1454. https://doi.org/10.3390/polym11091454
APA StyleChang, Y. -L., Hsieh, C. -Y., Yeh, C. -Y., & Lin, F. -H. (2019). The Development of Gelatin/Hyaluronate Copolymer Mixed with Calcium Sulfate, Hydroxyapatite, and Stromal-Cell-Derived Factor-1 for Bone Regeneration Enhancement. Polymers, 11(9), 1454. https://doi.org/10.3390/polym11091454