Molecularly Imprinted Nanoparticles Assay (MINA) in Pseudo ELISA: An Alternative to Detect and Quantify Octopamine in Water and Human Urine Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
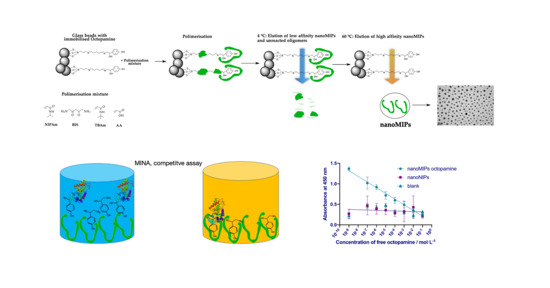
2.2. Preparation of Solid-Phase for Octopamine
2.3. Synthesis and Purification of NanoMIPs-O
2.4. Characterisation of NanoMIPs
2.5. Devolpment of MINA
2.5.1. Preparation of HRP-Octopamine (HRP-O) Conjugate
2.5.2. Immobilization of NanoMIPs onto the Surface of Microplate Wells
2.5.3. Optimisation of MINA Conditions
2.5.4. Optimisation of HRP-O Conjugate Concentration
2.5.5. Optimisation of NanoMIP Concentration
2.5.6. Competitive MINA for the Determination of Octopamine
2.5.7. Analysis of Octopamine in Human Urine Samples
2.5.8. Cross-Reactivity of the MINA Assay for Octopamine
3. Results and Discussion
3.1. Synthesis and Characterisation of NanoMIPs
3.2. Development of MINA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vieira, A. Elisa-basedassayfor scatchardanalysis of ligand-receptor interactions. Mol. Biotechnol. 1998, 10, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Lequin, R.M. Enzyme immunoassay (eia)/enzyme-linked immunosorbent assay (elisa). Clin. Chem. 2005, 51, 2415–2427. [Google Scholar] [CrossRef] [PubMed]
- Anantha-Iyengar, G.; Shanmugasundaram, K.; Nallal, M.; Lee, K.-P.; Whitcombe, M.J.; Lakshmi, D.; Sai-Anand, G. Functionalized conjugated polymers for sensing and molecular imprinting applications. Prog. Polym. Sci. 2019, 88, 1–129. [Google Scholar] [CrossRef]
- Haupt, K.M. Molecularly imprinted polymers and their use in biomimetic sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Wackerlig, J.; Schirhagl, R. Applications of molecularly imprinted polymer nanoparticles and their advances toward industrial use: A review. Anal. Chem. 2016, 88, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Ekpenyong-Akiba, A.E.; Canfarotta, F.; Abd, H.B.; Poblocka, M.; Casulleras, M.; Castilla-Vallmanya, L.; Kocsis-Fodor, G.; Kelly, M.E.; Janus, J.; Althubiti, M.; et al. Detecting and targeting senescent cells using molecularly imprinted nanoparticles. Nanoscale Horiz. 2019, 4, 757–768. [Google Scholar] [CrossRef]
- Cáceres, C.; Bravo, C.; Rivas, B.; Moczko, E.; Sáez, P.; García, Y.; Pereira, E. Molecularly imprinted polymers for the selective extraction of bisphenol a and progesterone from aqueous media. Polymers 2018, 10, 679. [Google Scholar] [CrossRef]
- Wulff, G.; Sarhan, A. Über die Anwendung von enzymanalog gebauten Polymeren zur Racemattrennung. Angew. Chem. Int. Ed. 1972, 11, 364–368. [Google Scholar] [CrossRef]
- Vlatakis, G.; Andersson, L.I.; Müller, R.; Mosbach, K. Drug assay using antibody mimics made by molecular imprinting. Nature 1993, 361, 645–647. [Google Scholar] [CrossRef]
- Mayes, A.G.; Whitcombe, M.J. Synthetic strategies for the generation of molecularly imprinted organic polymers. Adv. Drug Deliv. Rev. 2005, 57, 1742–1754. [Google Scholar] [CrossRef]
- Wang, J.; Dai, J.; Xu, Y.; Dai, X.; Zhang, Y.; Shi, W.; Sellergren, B.; Pan, G. Molecularly imprinted fluorescent test strip for direct, rapid, and visual dopamine detection in tiny amount of biofluid. Small 2019, 15, 1803913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Molecularly imprinted nanoparticles for biomedical applications. Adv. Mater. 2019, 1806328. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Chen, W.; Ma, Y.; Pan, G. Molecularly imprinted polymers as receptor mimics for selective cell recognition. Chem. Soc. Rev. 2018, 47, 5574–5587. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Dzgoev, A.; Mosbach, K. Assay system for the herbicide 2,4-dichlorophenoxyacetic acid using a molecularly imprinted polymer as an artificial recognition element. Anal. Chem. 1998, 70, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Bossi, A.; Piletsky, S.A.; Piletska, E.V.; Righetti, P.G.; Turner, A.P.F. Surface-grafted molecularly imprinted polymers for protein recognition. Anal. Chem. 2001, 73, 5281–5286. [Google Scholar] [CrossRef] [PubMed]
- Piletsky, S.A.; Piletska, E.V.; Chen, B.; Karim, K.; Weston, D.; Barrett, G.; Lowe, P.; Turner, A.P.F. Chemical grafting of molecularly imprinted homopolymers to the surface of microplates. Application of artificial adrenergic receptor in enzyme-linked assay for β-agonists determination. Anal. Chem. 2000, 72, 4381–4385. [Google Scholar] [CrossRef] [PubMed]
- Piletsky, S.A.; Piletska, E.V.; Bossi, A.; Karim, K.; Lowe, P.; Turner, A.P.F. Substitution of antibodies and receptors with molecularly imprinted polymers in enzyme-linked and fluorescent assays. Biosens. Bioelectron. 2001, 16, 701–707. [Google Scholar] [CrossRef] [Green Version]
- Surugiu, I.; Ye, L.; Yilmaz, E.; Dzgoev, A.; Danielsson, B.; Mosbach, K.; Haupt, K. An enzyme-linked molecularly imprinted sorbent assay. Analyst 2000, 125, 13–16. [Google Scholar] [CrossRef]
- Lakshmi, D.; Akbulut, M.; Ivanova-Mitseva, P.K.; Whitcombe, M.J.; Piletska, E.V.; Karim, K.; Güven, O.; Piletsky, S.A. Computational design and preparation of mips for atrazine recognition on a conjugated polymer-coated microtiter plate. Ind. Eng. Chem. Res. 2013, 52, 13910–13916. [Google Scholar] [CrossRef]
- Pardeshi, S.; Singh, S.K. Precipitation polymerization: A versatile tool for preparing molecularly imprinted polymer beads for chromatography applications. RSC Adv. 2016, 6, 23525–23536. [Google Scholar] [CrossRef]
- Poma, A.; Guerreiro, A.; Whitcombe, M.J.; Piletska, E.V.; Turner, A.P.F.; Piletsky, S.A. Solid-phase synthesis of molecularly imprinted polymer nanoparticles with a reusable template—“plastic antibodies”. Adv. Funct. Mater. 2013, 23, 2821–2828. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, A.R.; Chianella, I.; Piletska, E.; Whitcombe, M.J.; Piletsky, S.A. Selection of imprinted nanoparticles by affinity chromatography. Biosens. Bioelectron. 2009, 24, 2740–2743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poma, A.; Turner, A.P.F.; Piletsky, S.A. Advances in the manufacture of mip nanoparticles. Trends Biotechnol. 2010, 28, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Cenci, L.; Piotto, C.; Bettotti, P.; Maria Bossi, A. Study on molecularly imprinted nanoparticle modified microplates for pseudo-elisa assays. Talanta 2018, 178, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Moczko, E.; Guerreiro, A.; Cáceres, C.; Piletska, E.; Sellergren, B.; Piletsky, S.A. Epitope approach in molecular imprinting of antibodies. J. Chromatogr. B 2019, 1124, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, C.; Canfarotta, F.; Chianella, I.; Pereira, E.; Moczko, E.; Esen, C.; Guerreiro, A.; Piletska, E.; Whitcombe, M.J.; Piletsky, S.A. Does size matter? Study of performance of pseudo-elisas based on molecularly imprinted polymer nanoparticles prepared for analytes of different sizes. Analyst 2016, 141, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Merlier, F.; Avalle, B.; Vieillard, V.; Debré, P.; Haupt, K.; Tse Sum Bui, B. Molecularly imprinted polymer nanoparticles as potential synthetic antibodies for immunoprotection against hiv. ACS Appl. Mater. Interfaces 2019, 11, 9824–9831. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, Y.; Oino, D.; Ohira, H.; Muguruma, H.; Moczko, E.; Piletsky, A.S. Size of heparin-imprinted nanoparticles reflects the matched interactions with the target molecule. Sensors 2019, 19, 2415. [Google Scholar] [CrossRef]
- Chianella, I.; Guerreiro, A.; Moczko, E.; Caygill, J.S.; Piletska, E.V.; De Vargas Sansalvador, I.M.; Whitcombe, M.J.; Piletsky, S.A. Direct replacement of antibodies with molecularly imprinted polymer nanoparticles in elisa—Development of a novel assay for vancomycin. Anal. Chem. 2013, 85, 8462–8472. [Google Scholar] [CrossRef]
- Mattsson, L.; Xu, J.; Preininger, C.; Tse Sum Bui, B.; Haupt, K. Competitive fluorescent pseudo-immunoassay exploiting molecularly imprinted polymers for the detection of biogenic amines in fish matrix. Talanta 2018, 181, 190–196. [Google Scholar] [CrossRef]
- Smolinska-Kempisty, K.; Guerreiro, A.; Canfarotta, F.; Cáceres, C.; Whitcombe, M.J.; Piletsky, S. A comparison of the performance of molecularly imprinted polymer nanoparticles for small molecule targets and antibodies in the elisa format. Sci. Rep. 2016, 6, 37638. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J. Physiological functions and pharmacological and toxicological effects of p-octopamine. Drug Chem. Toxicol. 2015, 38, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Erspamer, V. Active substances in the posterior salivary glands of octopoda. Ii. Tyramine and octopamine (oxyoctopamine). Acta Pharmacol. Toxicol. 1948, 4, 224–247. [Google Scholar] [CrossRef]
- Zucchi, R.; Chiellini, G.; Scanlan, T.S.; Grandy, D.K. Trace amine-associated receptors and their ligands. Br. J. Pharmacol. 2006, 149, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Hackney, A.C. Chapter 3—Stimulants. In Doping, Performance Enhancing Drugs, and Hormones in Sport; Hackney, A.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 25–36. [Google Scholar]
- Mitchell, G.A.; Dunnavan, G. Illegal use of β-adrenergic agonists in the united states. J. Anim. Sci. 1998, 76, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Sigmund, G.; Guddat, S.; Schänzer, W.; Thevis, M. Determination of selected stimulants in urine for sports drug analysis by solid phase extraction via cation exchange and means of liquid chromatography-tandem mass spectrometry. Eur. J. Mass Spectrom. 2008, 14, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Thevis, M.; Koch, A.; Sigmund, G.; Thomas, A.; Schänzer, W. Analysis of octopamine in human doping control samples. Biomed. Chromatogr. 2011, 26, 610–615. [Google Scholar] [CrossRef]
- MyBioSource. Available online: https://www.mybiosource.com/opm-human-elisa-kits/octopamine/37215 (accessed on 7 March 2019).
- Munawar, H.; Smolinska-Kempisty, K.; Cruz, A.G.; Canfarotta, F.; Piletska, E.; Karim, K.; Piletsky, S.A. Molecularly imprinted polymer nanoparticle-based assay (mina): Application for fumonisin b1 determination. Analyst 2018, 143, 3481–3488. [Google Scholar] [CrossRef]
- Hoshino, Y.; Kodama, T.; Okahata, Y.; Shea, K.J. Peptide imprinted polymer nanoparticles: A plastic antibody. J. Am. Chem. Soc. 2008, 130, 15242–15243. [Google Scholar] [CrossRef]






| Procedure | Required Solution |
|---|---|
| 1. NanoMIPs immobilisation | 45 μL of nanoMIPs 0.056 mg·mL−1 (24 h ambient temperature, dark). |
| 2. Washing | 0.01 mol·L−1 of PBS (2 × 250 μL) pH 7.2. |
| 3. Blocking agent | 0.1% of BSA and 1% of Tween 20 in 0.01 mol·L−1 (300 μL, 2 h). |
| 4. Washing | 0.01 mol·L−1 of PBS (3 × 250 μL) pH 7.2. |
| 5. Target and conjugate | 100 μL of HRP-O conjugate (1:1200) and the standard solution of free octopamine 1 h. |
| 6. Washing | 0.1% of BSA and 1% of Tween 20 in 0.01 mol·L−1 (3 × 300 μL). |
| 7. Substrate addition | 100 μL of TMB solution, 10 min. |
| 8. Stopping solution | 100 μL of 0.5 mol·L−1 H2SO4. |
| Sample | Spiked (μg·mL−1) | Found (μg·mL−1) | Recovery (%) |
|---|---|---|---|
| Drinkable water 1 | 0.08 | 0.0851 | 106.4 |
| Human urine 1 | 0.08 | 0.0774 | 96.8 |
| Drinkable water 2 | 0.5 | 0.4810 | 96.2 |
| Human urine 2 | 0.5 | 0.515 | 103 |
| Drinkable water 3 | 50 | 51.6 | 103.2 |
| Human urine 3 | 50 | 49.5 | 99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moczko, E.; Díaz, R.; Rivas, B.; García, C.; Pereira, E.; Piletsky, S.; Cáceres, C. Molecularly Imprinted Nanoparticles Assay (MINA) in Pseudo ELISA: An Alternative to Detect and Quantify Octopamine in Water and Human Urine Samples. Polymers 2019, 11, 1497. https://doi.org/10.3390/polym11091497
Moczko E, Díaz R, Rivas B, García C, Pereira E, Piletsky S, Cáceres C. Molecularly Imprinted Nanoparticles Assay (MINA) in Pseudo ELISA: An Alternative to Detect and Quantify Octopamine in Water and Human Urine Samples. Polymers. 2019; 11(9):1497. https://doi.org/10.3390/polym11091497
Chicago/Turabian StyleMoczko, Ewa, Richard Díaz, Bernabé Rivas, Camilo García, Eduardo Pereira, Sergey Piletsky, and César Cáceres. 2019. "Molecularly Imprinted Nanoparticles Assay (MINA) in Pseudo ELISA: An Alternative to Detect and Quantify Octopamine in Water and Human Urine Samples" Polymers 11, no. 9: 1497. https://doi.org/10.3390/polym11091497
APA StyleMoczko, E., Díaz, R., Rivas, B., García, C., Pereira, E., Piletsky, S., & Cáceres, C. (2019). Molecularly Imprinted Nanoparticles Assay (MINA) in Pseudo ELISA: An Alternative to Detect and Quantify Octopamine in Water and Human Urine Samples. Polymers, 11(9), 1497. https://doi.org/10.3390/polym11091497