PPG-Terminated Tetra-Carbamates as the Toughening Additive for Bis-A Epoxy Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PPG-Terminated Tetra-Carbamates
2.3. Preparation of Modified Epoxy Resin with Tetra-Carbamates
2.4. Characterization
3. Results and Discussion
3.1. FT-IR Analysis on PTMG and Prepared Tetra-Carbamates
3.2. Rheological Analysis on Tetra-Carbamates/Epoxy Blends and the Tetra-Carbamates/Epoxy/TETA Curing System
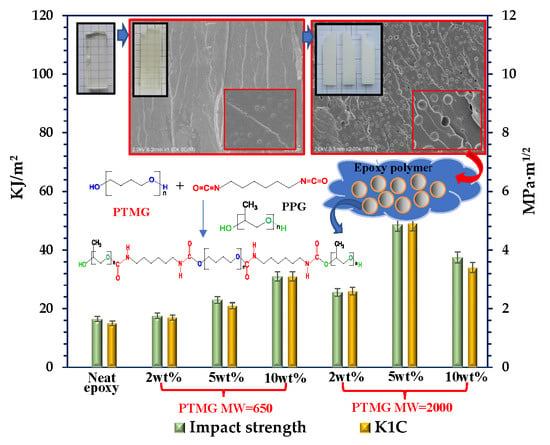
3.3. Cured Properties of Epoxy/TETA Modified with the Additives of Tetra-Carbamates
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, F.; Liu, W.; Zhou, M.; Li, W.; Hui, D.; Qiu, Y. Influenceo fcryogenic treatment on mechanical and interfacial properties of carbon nanotube fiber/bisphenol-F epoxy composite. Compos. B Eng. 2017, 125, 195–202. [Google Scholar] [CrossRef]
- May, C.A. Epoxy Resins; Marcel Dekker: New York, NY, USA, 1988. [Google Scholar]
- Wu, Z.X.; Huang, R.J.; Chu, X.X.; Huang, C.J.; Zhang, J.J.; Li, L.F. Cryogenic properties of hollow glass microsphere/epoxy composites. Adv. Cryog. Eng. AIP Conf. Procd. 2012, 1435, 156–163. [Google Scholar]
- Liu, S.; Chevali, V.S.; Xu, Z.; Hui, D.; Wang, H. A review of extending performance of epoxy resins using carbon nanomaterials. Compos. B Eng. 2018, 136, 197–214. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Hamim, S.U.; Karbalaei Akbari, M.; Fakhrhoseini, S.M.; Khayyam, H.; Pakseresht, A.H.; Ghasali, E.; Zabet, M.; Munir, K.S.; Jia, S.; et al. Carbon fibe rreinforced metal matrix composites: Fabricatio nprocesses and properties. Compos. Appl. Sci. Manuf. 2017, 92, 70–96. [Google Scholar] [CrossRef]
- Wang, L.L.; Tan, Y.F.; Wang, H.T.; Gao, L.; Zhou, C.X. Investigation on fracture behavior and mechanisms of DGEBF toughened by CTBN. Chem. Phys. Lett. 2018, 699, 14–21. [Google Scholar] [CrossRef]
- Wu, T.; Yu Liua, Y.; Lia, N.; Huang, G.W.; Qua, C.B.; Xiao, H.M. Cryogenic mechanical properties of epoxy resin toughened by hydroxyl-terminated polyurethane. Polym. Test. 2019, 74, 45–56. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Z.; Zhang, D. Synthesis and application of epoxy-ended hyperbranched polymers. Chem. Eng. J. 2018, 343, 283–302. [Google Scholar] [CrossRef]
- Lipic, P.; Bates, F.; Hillmyer, M. Nanostructured thermosets from self-assembled amphiphilic block copolymer/epoxy resin mixtures. J. Am. Chem. Soc. 1998, 120, 8963–8970. [Google Scholar] [CrossRef]
- Su, W.C.; Tsai, F.C.; Huang, C.F.; Dai, L.Z.; Kuo, S.W. Flexible epoxy resins formed by blending with the diblock copolymer PEO-b-PCL and using a hydrogen-bonding benzoxazine as the curing agent. Polymers 2019, 11, 201. [Google Scholar] [CrossRef]
- Builes, D.H.; Hernández-Ortiz, J.P.; Corcuera, M.A.; Mondragon, I.; Tercjak, A. Effect of poly(ethylene oxide) homopolymer and two different poly(ethylene oxide-b-poly(propylene oxide)-b-poly(ethylene oxide) triblock copolymers on morphological, optical, and mechanical properties of nanostructured unsaturated polyester. ACS Appl. Mater. Interfaces 2013, 6, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallego, M.; Verdejo, R.; Gestos, A.; Lopez-Manchado, M.A.; Guo, Q.P. Morphology and mechanical properties of nanostructured thermoset/block co-polymer blends with carbon nanoparticles. Compos. Part A 2015, 71, 136–143. [Google Scholar] [CrossRef]
- Guo, Q.P.; Thomann, R.; Gronski, W.; Thurn-Albrecht, T. Phase behavior, crystallization, and hierarchical nanostructures in self-organized thermoset blends of epoxy resin and amphiphilic poly (ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) triblock copolymers. Macromolecules 2002, 35, 3133–3144. [Google Scholar] [CrossRef]
- Bajpai, A.; Wetzel, B. Effect of different types of block copolymers on morphology, mechanical properties, and fracture mechanisms of bisphenol-F based epoxy system. J. Compos. Sci. 2019, 3, 68. [Google Scholar] [CrossRef]
- George, S.M.; Puglia, D.; Kenny, J.M.; Valerio Causin, V.; Parameswaranpillai, J.; Thomas, S. Morphological and mechanical characterization of nanostructured thermosets from epoxy and styrene-block-butadiene-block-styrene triblock copolymer. Ind. Eng. Chem. Res. 2013, 52, 9121–9129. [Google Scholar] [CrossRef]
- Varley, R.J. Toughening of epoxy resin systems using low-viscosity additives. Polym. Int. 2004, 53, 78–84. [Google Scholar] [CrossRef]
- Khanna, S.; Moniruzzaman, M.; Sundararajan, P.R. Influence of single versus double hydrogen-bonding motif on the crystallization and morphology of self-assembling carbamates with alkyl side chains: Model system for polyurethanes. J. Phys. Chem. B 2006, 110, 15251–15260. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Sundararajan, P.R. Role of hydrogen bonds in controlling the morphology of self-assembling carbamate systems. J. Phys. Chem. B 2013, 109, 1192–1197. [Google Scholar] [CrossRef]
- Fure, V.L. The IR spectra and hydrogen bonding of toluene-2,6-bis(methyl) and 4,4-prime diphenylmethane-bis(methyl) carbamates. J. Mol. Struct. 2000, 520, 117–123. [Google Scholar] [CrossRef]
- Ng, W.S.; Lee, C.S.; Cheng, S.F.; Chuah, C.H.; Wong, S.F. Biocompatible polyurethane scaffold sprepare dfro mglycero lmonostearate-derive dpolyeste rpolyol. J. Polym. Environ. 2018, 267, 2881–2900. [Google Scholar] [CrossRef]
- Michael, M.C.; Lee, K.H.; Skrovanek, D.J.; Painter, P.C. Hydrogen bonding in polymers. 4. Infrared temperature studies of a simple polyurethane. Macromolecules 1986, 19, 2149–2157. [Google Scholar]
- Khan, M.K.; Sundararajan, P.R. Effects of spacer length and terminal group on the crystallization and morphology of biscarbamates: A longer spacer does not reduce the melting temperature. J. Phys. Chem. B 2013, 117, 5705–5717. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Harrison, C.; Chaikin, P.M.; Register, R.A.; Adamson, R.A. Block copolymer lithography: Periodic arrays of ~1011 holes in 1 square centimeter. Science 1997, 276, 1401–1410. [Google Scholar] [CrossRef]
- Durga, G.; Narula, A.K.; Chin, J. Synthesis and characterization of diamide-diimide-diamines based on p-amino benzoic acid and their curing and thermal behavior with epoxy blends containing phosphorus/silicon in the main chain. Polym. Sci. 2012, 30, 694–701. [Google Scholar] [CrossRef]
- Ishida, H.; Allen, D.J. Mechanical characterization of copolymers based on benzoxazine and epoxy. Polymer 1996, 37, 4487–4492. [Google Scholar] [CrossRef]
- Marunaka, R.; Kawaguchi, M. Rheological behavior of hydrophobic fumed silica suspensions in aromatic dispersion media. J. Dispers. Sci. Technol. 2017, 38, 223–238. [Google Scholar] [CrossRef]
- Oh, T.K.; Hassan, M.; Beatty, C.; El-Shall, H. The effect of shear forces on the microstructure and mechanical properties of epoxy-clay nanocomposites. J. Appl. Polym. Sci. 2006, 100, 3465–3470. [Google Scholar] [CrossRef]
- Ghasemi, H.; Carreau, P.J.; Kama, l.M.R.; Tabatabaei, S.H. Properties of PET/clay nanocomposite films. Polym. Eng. Sci. 2012, 52, 420–430. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kim, J.C. Study on the Silicate Dispersion and Rheological Properties of PP/Starch-MB/Silicate Composites. J. Ind. Eng. Chem. 2007, 13, 1029–1034. [Google Scholar]
- Zhang, C.Y.; Zheng, S.X. Morphology and Fracture Toughness of Nanostructured Epoxy Resin Containing Amino-Terminated Poly(propylene oxide). J. Macromol. Sci. Part B Phys. 2010, 49, 574–591. [Google Scholar] [CrossRef]
- Boogh, L.; Pettersson, B.; Manson, J.E. Dendritic hyperbranched polymers as tougheners for epoxy resins. Polymer 1999, 40, 2249–2253. [Google Scholar] [CrossRef]
- Xie, X.L.; Tjong, S.C.; Li, R.K.Y. Study on in situ reinforcing and toughening of a semiflexible thermotropic copolyesteramide in PBT/PA66 blends. J. Appl. Polym. Sci. 2000, 77, 1957–1969. [Google Scholar] [CrossRef]
- Wang, H.H.; Chen, J.C. Toughening of Epoxy Resin by Functional-Terminated Polyurethanes and/or Semicrystalline Polymer Powders. J. Appl. Polym. Sci. 2001, 82, 2903–2912. [Google Scholar] [CrossRef]
- Wu, J.X.; Thio, Y.S.; Bates, F.S. Structure and Properties of PBO–PEO Diblock Copolymer Modified Epoxy. J. Polym. Sci. Part B Polym. Chem. 2005, 43, 1950–1965. [Google Scholar] [CrossRef]
- Misaki, T.; Hirohata, T.; Yoshi, M. Properties of Networks Obtained by Internal Plasticization of Epoxy Resin with Aromatic and Aliphatic Glycidyl Compounds. J. Appl. Polym. Sci. 1989, 37, 2617–2625. [Google Scholar] [CrossRef]
- Fu, J.F.; Shi, L.Y.; Yuan, S.; Zhong, Q.D.; Zhang, D.S.; Chen, Y.; Wu, J. Morphology, toughness mechanism, and thermal properties of hyperbranched epoxy modified diglycidyl ether of bisphenol A (DGEBA) interpenetrating polymer networks. Polym. Adv. Technol. 2008, 19, 1597–1607. [Google Scholar] [CrossRef]
- Parameswaranpillai, J.; Sidhardhan, S.K.; Harikrishnan, P.; Pionteck, J.; Siengchin, S.; Unni, A.B.; Magueresse, A.; Grohens, Y.; Hameed, N.; Jose, S. Morphology, thermo-mechanical properties and surface hydrophobicity of nanostructured epoxy thermosets modified with PEO-PPO-PEO triblock copolymer. Polym. Test. 2017, 59, 168–176. [Google Scholar] [CrossRef]
- Ratna, D.; Varley, D.; Raman, R.K.; Simon, G.P.; Ratna, S.R. Studies on blends of epoxy-functionalized hyperbranched polymer and epoxy resin. J. Mater. Sci. 2003, 38, 147–154. [Google Scholar] [CrossRef]




| Sample Name | Isocyanate | Dihydric Alcohol A | Dihydric Alcohol B |
|---|---|---|---|
| HT1P4 | HDI | PTMG650 | PPG400 |
| HT2P4 | HDI | PTMG2000 | PPG400 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Chen, M.; Ni, Z. PPG-Terminated Tetra-Carbamates as the Toughening Additive for Bis-A Epoxy Resin. Polymers 2019, 11, 1522. https://doi.org/10.3390/polym11091522
Zhang M, Chen M, Ni Z. PPG-Terminated Tetra-Carbamates as the Toughening Additive for Bis-A Epoxy Resin. Polymers. 2019; 11(9):1522. https://doi.org/10.3390/polym11091522
Chicago/Turabian StyleZhang, Ming, Mingqing Chen, and Zhongbin Ni. 2019. "PPG-Terminated Tetra-Carbamates as the Toughening Additive for Bis-A Epoxy Resin" Polymers 11, no. 9: 1522. https://doi.org/10.3390/polym11091522
APA StyleZhang, M., Chen, M., & Ni, Z. (2019). PPG-Terminated Tetra-Carbamates as the Toughening Additive for Bis-A Epoxy Resin. Polymers, 11(9), 1522. https://doi.org/10.3390/polym11091522