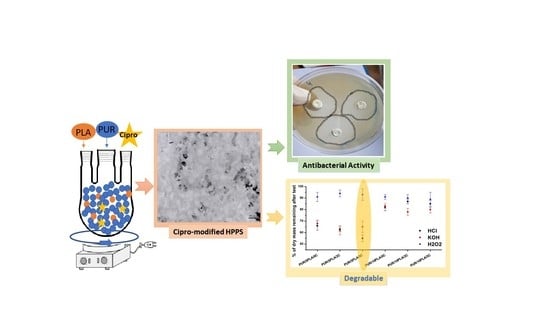
Ciprofloxacin-Modified Degradable Hybrid Polyurethane-Polylactide Porous Scaffolds Developed for Potential Use as an Antibacterial Scaffold for Regeneration of Skin
Abstract
:1. Introduction
2. Materials
3. Methods
3.1. Fabrication of Porous Hybrid Polyurethane-Polyester Porous Scaffolds (HPPS)
3.2. Modification of HPPS with an Antibacterial Agent from the Group of Fluoroquinolones
3.3. Samples, Symbols, and Ratios
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.5. Mechanical Properties
3.6. Optical Microscopy
3.7. Scanning Electron Microscopy (SEM)
3.8. Short-Term Degradation Studies in Selected Media
3.9. Microbiological Tests
4. Results
4.1. Fourier-Transform Infrared Spectroscopy
4.2. Mechanical Properties
4.3. Scanning Electron Microscopy
4.4. Short-Term Interaction with Selected Media
4.5. Microbiological Tests
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- Esteban-vives, R.; Young, M.T.; Ziembicki, J.; Corcos, A.; Gerlach, C. Effects of wound dressings on cultured primary keratinocytes. Burns 2016, 42, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Wohlsein, P.; Peters, M.; Schulze, C.; Baumga, W. Thermal Injuries in Veterinary Forensic Pathology. Vet. Pathol. 2016, 53, 1001–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Evans, D. Tissue engineering: The future of stem cells. Top. Tissue Eng. 2005, 2, 1–22. [Google Scholar]
- Brekke, J.H.; Toth, J.M. Principles of tissue engineering applied to programmable osteogenesis. J. Biomed. Mater. Res. 1998, 43, 380–398. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Callaghan, M.J.; Longaker, M.T. Progress and potential for regenerative medicine. Annu. Rev. Med. 2007, 58, 299–312. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314. [Google Scholar] [CrossRef]
- Feinberg, A.W. Engineered tissue grafts: Opportunities and challenges in regenerative medicine. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 207–220. [Google Scholar] [CrossRef]
- Kucinska-Lipka, J.; Gubanska, I.; Janik, H.; Pokrywczynska, M.; Drewa, T. L-ascorbic acid modified poly(ester urethane)s as a suitable candidates for soft tissue engineering applications. React. Funct. Polym. 2015, 97, 105–115. [Google Scholar] [CrossRef]
- Lipka, J.K.; Lewandowska, I.G.A. Antibacterial polyurethanes, modified with cinnamaldehyde, as potential materials for fabrication of wound dressings. In Polymer Bulletin; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Kucińska-Lipka, J.; Gubanska, I.; Skwarska, A. Microporous polyurethane thin layer as a promising scaffold for tissue engineering. Polymers 2017, 9, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heureux, L.; Fricain, J.; Catros, S.; Le Nihouannen, D. Characterization of printed PLA scaffolds for bone tissue engineering. J. Biolmed. Mater. Res. 2018, 106, 887–894. [Google Scholar]
- Li, L.; Li, Q.; Yang, J.; Sun, L.; Guo, J.; Yao, Y.; Zhong, L.; Li, D. Enhancement in mechanical properties and cell activity of polyurethane scaffold derived from gastrodin. Mater. Lett. 2018, 228, 435–438. [Google Scholar] [CrossRef]
- Mi, H.; Jing, X.; Yu, E.; Wang, X.; Li, Q.; Turng, L. Manipulating the structure and mechanical properties of thermoplastic polyurethane/polycaprolactone hybrid small diameter vascular scaff olds fabricated via electrospinning using an assembled rotating collector. J. Mech. Behav. Biomed. Mater. 2018, 78, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.P.; Sell, S.A.; Boland, E.D.; Simpson, D.G.; Bowlin, G.L. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv. Drug Deliv. Rev. 2007, 59, 1413–1433. [Google Scholar] [CrossRef] [PubMed]
- Gubanska, I.; Kucinska-Lipka, J.; Janik, H. The influence of amorphous macrodiol, diisocyanate type and L-ascorbic acid modifier on chemical structure, morphology and degradation behavior of polyurethanes for tissue scaffolds fabrication. In Polymer Degradation and Stability; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 163, pp. 52–67. [Google Scholar]
- Mikos, A.G.; Herring, S.W.; Ochareon, P.; Elisseeff, J.; Lu, H.H.; Kandel, R.; Schoen, F.J.; Toner, M.; Mooney, D.; Atala, A.; et al. Engineering complex tissues. Tissue Eng. 2006, 12, 3307–3339. [Google Scholar] [CrossRef] [PubMed]
- Palmiero, C.; Imparato, G.; Urciuolo, F.; Netti, P. Engineered dermal equivalent tissue in vitro by assembly of microtissue precursors. Acta Biomater. 2010, 6, 2548–2553. [Google Scholar] [CrossRef]
- Urciuolo, F.; Imparato, G.; Totaro, A. Building a tissue in vitro from the bottom up: Implications in regenerative medicine. Methodist Debakey Cardiovasc. J. 2013, 9, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.B.; Mauck, R.L. Tissue Engineering and Regenerative Medicine: Recent Innovations and the Transition to Translation. Tissue Eng. Part B Rev. 2013, 19, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Li, Y.; Zou, Q. Degradation and biocompatibility of porous nano-hydroxyapatite/polyurethane composite scaffold for bone tissue engineering. Appl. Surf. Sci. 2009, 255, 6087–6091. [Google Scholar] [CrossRef]
- Tatai, L.; Moore, T.G.; Adhikari, R. Thermoplastic biodegradable polyurethanes: The effect of chain extender structure on properties and in-vitro degradation. Biomaterials 2007, 28, 5407–5417. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Gustafson, C.T.; Lee, A.; Ii, M.; Waletzki, B.E.; Yaszemski, M.J.; Lu, L. Tunable tissue sca ff olds fabricated by in situ crosslink in phase separation system. RSC Adv. R. Soc. Chem. 2015, 5, 100824–100833. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- DW, H. scaffold-based bone engineering by using rapi prototyping technologies in virtual and rapid manufacturing. In Advanced Research in Virtual and Rapid Prototyping; Bartolo, J.B., Ed.; Taylor & Francis Group: Abingdon-on-Thames, UK, 2008; p. 65. [Google Scholar]
- Guelcher, S.A.; Srinivasan, A.; Dumas, J.E.; Didier, J.E.; McBride, S.; Hollinger, J.O. Synthesis, mechanical properties, biocompatibility, and biodegradation of polyurethane networks from lysine polyisocyanates. Biomaterials 2008, 29, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Montini-Ballarin, F.; Caracciolo, P.C.; Rivero, G.; Abraham, G.A. In vitro degradation of electrospun poly(l-lactic acid)/segmented poly(ester urethane) blends. Polym. Degrad. Stab. 2016, 126, 159–169. [Google Scholar] [CrossRef]
- Ballarin, F.M.; Caracciolo, P.C.; Blotta, E.; Ballarin, V.L.; Abraham, G.A. Optimization of poly(L-lactic acid)/segmented polyurethane electrospinning process for the production of bilayered small-diameter nano fi brous tubular structures. Mater. Sci. Eng. C 2014, 42, 489–499. [Google Scholar] [CrossRef]
- Gudiño-rivera, J.; Medellín-rodríguez, F.J.; Ávila-orta, C.; Palestino-escobedo, A.G.; Sánchez-valdés, S. Structure/property relationships of poly(L-lactic acid)/mesoporous silica nanocomposites. J. Polym. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. Part. B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [Green Version]
- Lipsa, R.; Tudorachi, N.; Vasile, C. Poly(α-hydroxyacids ) in biomedical applications: Synthesis and properties of lactic acid polymers. e-Polymers 2010, 10. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Maciel, R. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Vats, A.; Tolley, Ã.N.S.; Polak, J.M.Ã.; Gough, J.E.Ã. Scaffolds and biomaterials for tissue engineering: A review of clinical applications. Clin. Otolaryngol. Allied Sci. 2003, 28, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, M.A.; Kim, K.; Park, J.; Deep, A. Hydrolytic degradation of polylactic acid(PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Adhikari, R.; Scientific, T.C. Biodegradable polyurethanes: Design, synthesis, properties and potential applications. In Biodegradable Polymers: Processing, Degradation and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 431–470. [Google Scholar]
- Guelcher, S.A. Biodegradable polyurethanes: Synthesis and applications in regenerative medicine. Tissue Eng. Part. B Rev. 2008, 14, 11–19. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Kwok, C.S.; Wan, C.; Hendricks, S.; Bryers, J.D.; Horbett, T.A.; Ratner, B.D. Design of infection-resistant antibiotic-releasing polymers: I. fabrication and formulation. J. Control. Release 1999, 62, 289–299. [Google Scholar] [CrossRef]
- Field, K.; Kerstein, M.D. Overview of wound healing in a moist environment. Am. J. Surg. 1994, 167, 2–6. [Google Scholar] [CrossRef]
- Anjum, S.; Arora, A.; Alam, M.S.; Gupta, B. Development of antimicrobial and scar preventive chitosan hydrogel wound dressings. Int. J. Pharm. 2016, 508, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Vowden, K. Wound dressings: Principles and practice. In Surgery; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 1–6. [Google Scholar]
- Koosehgol, S.; Ebrahimian-hosseinabadi, M.; Alizadeh, M.; Zamanian, A. Preparation and characterization of in situ chitosan/polyethylene glycol fumarate/thymol hydrogel as an effective wound dressing. Mater. Sci. Eng. C 2017, 79, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Yari, A.; Yeganeh, H.; Bakhshi, H. Synthesis and evaluation of novel absorptive and antibacterial polyurethane membranes as wound dressing. J. Mater. Sci. Mater. Med. 2012, 23, 2187–2202. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, R.; Buzatto, C.; Alberto, J.; Maria, Â. Electrospun multilayer chitosan scaffolds as potential wound dressings for skin lesions. Eur. Polym. J. 2017, 88, 161–170. [Google Scholar]
- Sikareepaisan, P.; Ruktanonchai, U.; Supaphol, P. Preparation and characterization of asiaticoside-loaded alginate films and their potential for use as effectual wound dressings. Carbohydr. Polym. 2011, 83, 1457–1469. [Google Scholar] [CrossRef]
- Unnithan, A.R.; Barakat, N.A.M.; Pichiah, P.B.T.; Gnanasekaran, G.; Nirmala, R.; Cha, Y.; Jung, C.H.; El-Newehy, M.; Kim, H.Y. Wound-dressing materials with antibacterial activity from electrospun polyurethane—dextran nanofiber mats containing ciprofloxacin HCl. Carbohydr. Polym. 2012, 90, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Nagarwal, R.C.; Kant, S.; Singh, P.N.; Maiti, P.; Pandit, J.K. Polymeric nanoparticulate system: A potential approach for ocular drug delivery. J. Control. Release 2009, 136, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.; Banik, R.M. Development of ciprofloxacin hydrochloride loaded poly(ethylene glycol)/chitosan scaffold as wound dressing. J. Porous Mater. 2013, 20, 799–807. [Google Scholar] [CrossRef]
- Bergman, B.; Bishop, M.C.; Bjerklund-johansen, T.E.; Botto, H.; Lobel, B.; Cruz, F.J.; Selvaggi, F.P. EAU guidelines for the management of urinary and male genital tract infectionstextsuperscript1. Eur. Urol. 2001, 40, 576–588. [Google Scholar]
- Zeiler, H.; Grohe, K.; Ag, B.; Ciprofloxacin, A.A. the in vitro and in vivo activity of ciprofloxacin. In Ciprofloxacin; Vieweg+Teubner Verlag: Wiesbaden, Germany, 1986; pp. 14–18. [Google Scholar]
- Dillen, K.; Vandervoort, J.; Van Den Mooter, G.; Verheyden, L.; Ludwig, A. Factorial design, physicochemical characterisation and activity of ciprofloxacin-PLGA nanoparticles. Int. J. Pharm. 2004, 275, 171–187. [Google Scholar] [CrossRef]
- Page, J.M.; Prieto, E.M.; Dumas, J.E.; Zienkiewicz, K.J.; Wenke, J.C.; Brown-Baer, P.; Guelcher, S.A. Biocompatibility and chemical reaction kinetics of injectable, settable polyurethane/allograft bone biocomposites. Acta Biomater. 2012, 8, 4405–4416. [Google Scholar] [CrossRef]
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. 2015, 12, 47–52. [Google Scholar] [CrossRef]
- Boffito, M.; Sartori, S.; Ciardelli, G. Polymeric scaffolds for cardiac tissue engineering: Requirements and fabrication technologies. Polym. Int. 2014, 63, 2–11. [Google Scholar] [CrossRef]
- Janik, H.; Marzec, M. A review: Fabrication of porous polyurethane scaffolds. Mater. Sci. Eng. C 2015, 48, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, A.; Boffito, M.; Sartori, S.; Ciardelli, G. Biomimetic materials and scaffolds for myocardial tissue regeneration. Macromol. Biosci. 2013, 13, 984–1019. [Google Scholar] [CrossRef]
- Stachelek, S.J.; Alferiev, I.; Ueda, M.; Eckels, E.C.; Kevin, T.; Levy, R.J. Prevention of polyurethane oxidative degradation with phenolic-antioxidants covalently attached to the hard segments: Structure function. J. Biomed. Mater. Res. Part. A 2010, 94, 751–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cetina-Diaz, S.M.; Chan-Chan, L.H.; Vargas-Coronado, R.F.; Cervantes-Uc, J.M.; Quintana-Owen, P.; Paakinaho, K.; Kellomaki, M.; Silvio, L.D.; Deb, S.; Cauich-Rodríguez, J.V. Physicochemical characterization of segmented polyurethanes prepared with glutamine or ascorbic acid as chain extenders and their hydroxyapatite composites. J. Mater. Chem. B 2014, 2, 1966–1976. [Google Scholar] [CrossRef]
- Tan, Z.; Tan, F.; Zhao, L.; Li, J. The Synthesis, Characterization and Application of Ciprofloxacin Complexes and Its Coordination with Copper, Manganese and Zirconium Ions. J. Cryst. Process Technol. 2012, 2, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Yilgor, I.; Yilgor, E.; Guler, I.G.; Ward, T.C.; Wilkes, G.L. FTIR investigation of the influence of diisocyanate symmetry on the morphology development in model segmented polyurethanes. Polymer 2006, 47, 4105–4114. [Google Scholar] [CrossRef]
- Doolittle, J.; Su, H.-C.; Khatun, J.; Secrest, A.; Clark, M.; Ramkissoon, K.; Wolfgang, M.C.; Giddings, M.C. The development of ciprofloxacin resistance in pseudomonas aeruginosa involves multiple response stages and multiple proteins. Antimicrob. Agents Chemother. 2010, 54, 4626–4635. [Google Scholar]
- Kucinska-Lipka, J.; Gubanska, I.; Sienkiewicz, M. Thermal and mechanical properties of polyurethanes modified with L-ascorbic acid. J. Therm. Anal. Calorim. 2017, 127, 1631–1638. [Google Scholar] [CrossRef] [Green Version]
- Diridollou, S.; Patat, F.; Gens, F.; Vaillant, L.; Black, D.; Lagarde, J.M.; Gall, Y.; Berson, M. In vivo model of the mechanical properties of the human skin under suction. Skin Res. Technol. 2000, 6, 214–221. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Ní Anniadh, A.; Bruyère, K.; Otténio, M.; Xie, H.; Gilchrist1, M.D. Dynamic tensile properties of human skin. In Proceedings of the 2012 IRCOBI Conference International Research Council on the Biomechanics of Injury, Dublin, Ireland, 12–14 Sepember 2012; pp. 494–502. [Google Scholar]
- Kucińska-Lipka, J.; Gubańska, I.; Janik, H. Gelatin-modified polyurethanes for soft tissue scaffold. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]









| Symbol | Explanation | Ratios | |||
|---|---|---|---|---|---|
| PUR | PLA | CIPRO | GELATIN | ||
| PUR/10PLA/0C | Unmodified HPPS obtained with 10 wt % of PLA, not modified with Cipro | 17 | 2 | 0 | 1 |
| PUR/10PLA/2C | HPPS obtained with 10 wt % of PLA, modified with 2 wt % of Cipro | 41.5 | 5 | 1 | 2.5 |
| PUR/10PLA/5C | HPPS obtained with 10 wt % of PLA, modified with 5 wt % of Cipro | 16 | 2 | 1 | 1 |
| PUR/5PLA/0C | Unmodified HPPS obtained with 5 wt % of PLA, not modified with Cipro | 18 | 1 | 0 | 1 |
| PUR/5PLA/2C | HPPS obtained with 5 wt % of PLA, modified with 2 wt % of Cipro | 44 | 2.5 | 1 | 2.5 |
| PUR/5PLA/5C | HPPS obtained with 5 wt % of PLA, modified with 5 wt % of Cipro | 17 | 1 | 1 | 1 |
| Symbol | Porosity (%) |
|---|---|
| PUR/5PLA/0C | 86% |
| PUR/5PLA/2C | 87% |
| PUR/5PLA/5C | 85% |
| PUR/10PLA/0C | 84% |
| PUR/10PLA/2C | 72% |
| PUR/10PLA/5C | 64% |
| Symbol | Inhibition Zone (mm) |
|---|---|
| PUR/5PLA/0C | 0 |
| PUR/5PLA/2C | 15 |
| PUR/5PLA/5C | 20 |
| PUR/10PLA/0C | 0 |
| PUR/10PLA/2C | 16 |
| PUR/10PLA/5C | 22 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iga, C.; Agata, T.; Marcin, Ł.; Natalia, F.; Justyna, K.-L. Ciprofloxacin-Modified Degradable Hybrid Polyurethane-Polylactide Porous Scaffolds Developed for Potential Use as an Antibacterial Scaffold for Regeneration of Skin. Polymers 2020, 12, 171. https://doi.org/10.3390/polym12010171
Iga C, Agata T, Marcin Ł, Natalia F, Justyna K-L. Ciprofloxacin-Modified Degradable Hybrid Polyurethane-Polylactide Porous Scaffolds Developed for Potential Use as an Antibacterial Scaffold for Regeneration of Skin. Polymers. 2020; 12(1):171. https://doi.org/10.3390/polym12010171
Chicago/Turabian StyleIga, Carayon, Terebieniec Agata, Łapiński Marcin, Filipowicz Natalia, and Kucińska-Lipka Justyna. 2020. "Ciprofloxacin-Modified Degradable Hybrid Polyurethane-Polylactide Porous Scaffolds Developed for Potential Use as an Antibacterial Scaffold for Regeneration of Skin" Polymers 12, no. 1: 171. https://doi.org/10.3390/polym12010171
APA StyleIga, C., Agata, T., Marcin, Ł., Natalia, F., & Justyna, K. -L. (2020). Ciprofloxacin-Modified Degradable Hybrid Polyurethane-Polylactide Porous Scaffolds Developed for Potential Use as an Antibacterial Scaffold for Regeneration of Skin. Polymers, 12(1), 171. https://doi.org/10.3390/polym12010171