Preparation, Characterization and Application of a Low Water-Sensitive Artemisia sphaerocephala Krasch. Gum Intelligent Film Incorporated with Anionic Cellulose Nanofiber as a Reinforcing Component
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Regents
2.2. Experimental Methods
2.2.1. Extraction and Quantification of RCE
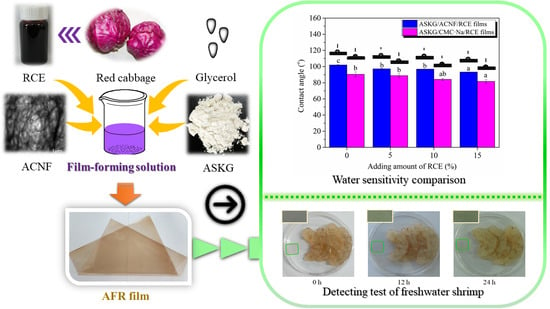
2.2.2. Preparation of ASKG/ACNF/RCE Intelligent Films
2.2.3. Preparation of Intelligent Films to Detect Freshwater Shrimp Freshness
2.3. Characterization
2.3.1. Zeta Potential Changes of Solutions
2.3.2. Rheological Analysis of Solutions
2.3.3. Fourier-Transform Infrared Spectroscopy
2.3.4. X-ray Diffractometry Spectroscopy
2.3.5. Thermogravimetric Analysis
2.3.6. Morphology Analysis
2.3.7. Mechanical Properties
2.3.8. Barrier Properties
2.3.9. Light Transmittance Analysis
2.3.10. Release Test
2.3.11. Water-Sensitivity Test of Intelligent Films
2.3.12. Chroma-Response Test
2.3.13. pH and Total Volatile Basic Nitrogen of Freshwater Shrimp
2.3.14. Freshness Detection Test
2.3.15. Statistical Treatment
3. Results and Discussions
3.1. Zeta Potential Analysis
3.2. Rheological Analysis
3.2.1. Steady Rheological Analysis
3.2.2. Dynamic Rheological Analysis
3.3. FT-IR Analysis
3.4. XRD Analysis
3.5. Thermogravimetric Analysis
3.6. TEM and SEM Observation
3.7. Mechanical Properties
3.8. Oxygen and Water Vapor Permeabilities
3.9. Light Transmission of Intelligent Films
3.10. Water-Sensitivity Analysis
3.11. Release Analysis
3.12. Buffer Solutions and NH3 Atmosphere Response Analysis of Intelligent Films
3.12.1. Chroma-Response in Buffer Solution
3.12.2. Chroma-Response in NH3 Condition
3.13. Detecting Analysis of Intelligent Film for Freshwater Shrimp Freshness in Real Time
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Oliveira, A.F.; Silveira, C.B.; Ernani, P.R.; Balbinot, E.S.; Soldi, V. Potassium ions release from polysaccharide films. J. Braz. Chem. Soc. 2011, 22, 211–216. [Google Scholar] [CrossRef]
- Fang, Z.; Zhao, Y.; Warner, R.D.; Johnson, S.K. Active and intelligent packaging in meat industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, X.; Qin, Y.R.; Zhang, Y.H.; Wang, X.P.; Wang, J.Y.; Ning, Z.X.; Ruan, Q.J.; Zhang, Y.S. Preparation and characterization of a novel colorimetric indicator film based on gelatin/polyvinyl alcohol incorporating mulberry anthocyanin extracts for monitoring fish freshness. Food Res. Int. 2019, 126, 108604. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Sun, J.; Chen, M.; Ge, Y.; Ma, J.; Hu, Y.; Pang, J.; Yan, Z. Effect of oxidized chitin nanocrystals and curcumin into chitosan films for seafood freshness monitoring. Food Hydrocoll. 2019, 95, 308–317. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Shi, J.; Huang, X.; Sun, Z.; Zhang, D.; Zou, X.; Sun, Y.; Zhang, J.; Holmes, M.; et al. A colorimetric hydrogen sulfide sensor based on gellan gum-silver nanoparticles bionanocomposite for monitoring of meat spoilage in intelligent packaging. Food Chem. 2019, 290, 135–143. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Gutiérrez-Tauste, D.; Domènech, X.; Casañ-Pastor, N.; Ayllón, J.A. Characterization of methylene blue/TiO2 hybrid thin films prepared by the liquid phase deposition (LPD) method: Application for fabrication of light-activated colorimetric oxygen indicators. J. Photochem. Photobiol. A 2007, 187, 45–52. [Google Scholar] [CrossRef]
- Makote, R.; Collinson, M.M. Organically modified silicate films for stable pH sensors. Anal. Chim. Acta 1999, 394, 195–200. [Google Scholar] [CrossRef]
- Dong, S.; Luo, M.; Peng, G.; Cheng, W. Broad range pH sensor based on sol–gel entrapped indicators on fibre optic. Sens. Actuators B 2008, 129, 94–98. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, S.; Chen, X. A visual pH sensing film using natural dyes from Bauhinia blakeana Dunn. Sens. Actuators B 2014, 198, 268–273. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Preparation of carbohydrate-based functional composite films incorporated with curcumin. Food Hydrocoll. 2020, 98, 105302. [Google Scholar] [CrossRef]
- Said, N.S.; Sarbon, N.M. Response surface methodology (RSM) of chicken skin gelatin based composite films with rice starch and curcumin incorporation. Polym. Test. 2019, 106161. [Google Scholar] [CrossRef]
- Da Nascimento Silva, M.; de Matos Fonseca, J.; Feldhaus, H.K.; Soares, L.S.; Valencia, G.A.; de Maduro Campos, C.E.; Di Luccio, M.; Monteiro, A.R. Physical and morphological properties of hydroxypropyl methylcellulose films with curcumin polymorphs. Food Hydrocoll. 2019, 97, 105217. [Google Scholar] [CrossRef]
- Koosha, M.; Hamedi, S. Intelligent Chitosan/PVA nanocomposite films containing black carrot anthocyanin and bentonite nanoclays with improved mechanical, thermal and antibacterial properties. Prog. Org. Coat. 2019, 127, 338–347. [Google Scholar] [CrossRef]
- Carvalho, V.V.L.; Goncalves, J.O.; Silva, A.; Cadaval, T.R., Jr.; Pinto, L.A.A.; Lopes, T.J. Separation of anthocyanins extracted from red cabbage by adsorption onto chitosan films. Int. J. Biol. Macromol. 2019, 131, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Li, Y.; Sun, J.; Lu, Y.; Tong, C.; Wang, L.; Yan, Z.; Pang, J. Novel konjac glucomannan films with oxidized chitin nanocrystals immobilized red cabbage anthocyanins for intelligent food packaging. Food Hydrocoll. 2020, 98, 105245. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Bai, R.; Miao, Z.; Zhang, X.; Liu, J. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, X.; Zhai, X.; Huang, X.; Jiang, C.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef]
- Won, K.; Jang, N.Y.; Jeon, J. A Natural Component-Based Oxygen Indicator with In-Pack Activation for Intelligent Food Packaging. J. Agric. Food Chem. 2016, 64, 9675–9679. [Google Scholar] [CrossRef]
- Krga, I.; Milenkovic, D. Anthocyanins: From Sources and Bioavailability to Cardiovascular-Health Benefits and Molecular Mechanisms of Action. J. Agric. Food Chem. 2019, 67, 1771–1783. [Google Scholar] [CrossRef]
- Silva-Pereira, M.C.; Teixeira, J.A.; Pereira-Júnior, V.A.; Stefani, R. Chitosan/corn starch blend films with extract from Brassica oleraceae (red cabbage) as a visual indicator of fish deterioration. LWT Food Sci. Technol. 2015, 61, 258–262. [Google Scholar] [CrossRef] [Green Version]
- Pourjavaher, S.; Almasi, H.; Meshkini, S.; Pirsa, S.; Parandi, E. Development of a colorimetric pH indicator based on bacterial cellulose nanofibers and red cabbage (Brassica oleraceae) extract. Carbohydr. Polym. 2017, 156, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Wang, L. Preparation and characterization of a novel edible film based on Artemisia sphaerocephala Krasch. gum: Effects of type and concentration of plasticizers. Food Hydrocoll. 2018, 77, 502–508. [Google Scholar] [CrossRef]
- Liang, T.; Sun, G.; Cao, L.; Li, J.; Wang, L. Rheological behavior of film-forming solutions and film properties from Artemisia sphaerocephala Krasch. gum and purple onion peel extract. Food Hydrocoll. 2018, 82, 124–134. [Google Scholar] [CrossRef]
- Liang, T.; Sun, G.; Cao, L.; Li, J.; Wang, L. A pH and NH3 sensing intelligent film based on Artemisia sphaerocephala Krasch. gum and red cabbage anthocyanins anchored by carboxymethyl cellulose sodium added as a host complex. Food Hydrocoll. 2019, 87, 858–868. [Google Scholar] [CrossRef]
- Jayakumar, A.; Heera, K.V.; Sumi, T.S.; Joseph, M.; Mathew, S.; Praveen, G.; Nair, I.C.; Radhakrishnan, E.K. Starch-PVA composite films with zinc-oxide nanoparticles and phytochemicals as intelligent pH sensing wraps for food packaging application. Int. J. Biol. Macromol. 2019, 136, 395–403. [Google Scholar] [CrossRef]
- Ebrahimi Tirtashi, F.; Moradi, M.; Tajik, H.; Forough, M.; Ezati, P.; Kuswandi, B. Cellulose/chitosan pH-responsive indicator incorporated with carrot anthocyanins for intelligent food packaging. Int. J. Biol. Macromol. 2019, 136, 920–926. [Google Scholar] [CrossRef]
- Andretta, R.; Luchese, C.L.; Tessaro, I.C.; Spada, J.C. Development and characterization of pH-indicator films based on cassava starch and blueberry residue by thermocompression. Food Hydrocoll. 2019, 93, 317–324. [Google Scholar] [CrossRef]
- Huq, T.; Salmieri, S.; Khan, A.; Khan, R.A.; Le Tien, C.; Riedl, B.; Fraschini, C.; Bouchard, J.; Uribe-Calderon, J.; Kamal, M.R.; et al. Nanocrystalline cellulose (NCC) reinforced alginate based biodegradable nanocomposite film. Carbohydr. Polym. 2012, 90, 1757–1763. [Google Scholar] [CrossRef]
- Atef, M.; Rezaei, M.; Behrooz, R. Preparation and characterization agar-based nanocomposite film reinforced by nanocrystalline cellulose. Int. J. Biol. Macromol. 2014, 70, 537–544. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, Y.; Chen, G.; Cai, M. Enhanced mechanical and hydrophobic properties of composite cassava starch films with stearic acid modified MCC (microcrystalline cellulose)/NCC (nanocellulose) as strength agent. Int. J. Biol. Macromol. 2020, 142, 846–854, in press. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Wang, W.; Teng, A.; Zhang, K.; Ma, Y.; Duan, S.; Li, S.; Guo, Y. Using cellulose nanofibers to reinforce polysaccharide films: Blending vs layer-by-layer casting. Carbohydr. Polym. 2020, 227, 115264. [Google Scholar] [CrossRef] [PubMed]
- Chinga-Carrasco, G. Cellulose fibres, nanofibrils and microfibrils: The morphological sequence of MFC components from a plant physiology and fibre technology point of view. Nanoscale Res. Lett. 2011, 6, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akindoyo, J.O.; Ismail, N.H.; Mariatti, M. Performance of poly (vinyl alcohol) nanocomposite reinforced with hybrid TEMPO mediated cellulose-graphene filler. Polym. Test. 2019, 80, 106140. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Fujisawa, S.; Saito, T.; Kimura, S.; Iwata, T.; Isogai, A. Surface engineering of ultrafine cellulose nanofibrils toward polymer nanocomposite materials. Biomacromolecules 2013, 14, 1541–1546. [Google Scholar] [CrossRef]
- Truong, V.D.; Hu, Z.; Thompson, R.L.; Yencho, G.C.; Pecota, K.V. Pressurized liquid extraction and quantification of anthocyanins in purple-fleshed sweet potato genotypes. J. Food Compos. Anal. 2012, 26, 96–103. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red cabbage anthocyanins: Profile, isolation, identification, and antioxidant activity. Food Res. Int. 2013, 51, 303–309. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Li, C.; Du, G.; Zhang, H.; Ni, Y. Using carboxylated cellulose nanofibers to enhance mechanical and barrier properties of collagen fiber film by electrostatic interaction. J. Sci. Food Agric. 2018, 98, 3089–3097. [Google Scholar] [CrossRef]
- Lin, H.Y.; Tsai, J.C.; Lai, L.S. Effect of salts on the rheology of hydrocolloids from mulberry (Morus alba L.) leaves in concentrated domain. Food Hydrocoll. 2009, 23, 2331–2338. [Google Scholar] [CrossRef]
- Haddarah, A.; Bassal, A.; Ismail, A.; Gaiani, C.; Ioannou, I.; Charbonnel, C.; Hamieh, T.; Ghoul, M. The structural characteristics and rheological properties of Lebanese locust bean gum. J. Food Eng. 2014, 120, 204–214. [Google Scholar] [CrossRef]
- Long, Z.; Zhao, M.; Zhao, Q.; Yang, B.; Liu, L. Effect of homogenisation and storage time on surface and rheology properties of whipping cream. Food Chem. 2012, 13, 748–753. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, W.; Jia, L.; He, Q. The rheological properties of tara gum (Caesalpinia spinosa). Food Chem. 2015, 168, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Islam, M.S.; Christopher, L.P. Sustainable Production of Cellulose-Based Hydrogels with Superb Absorbing Potential in Physiological Saline. ACS Omega 2019, 4, 9419–9426. [Google Scholar] [CrossRef]
- Yuan, H.; Guo, X.; Xiao, T.; Ma, Q.; Wu, Y. Moisture adsorption in TEMPO-oxidized cellulose nanocrystal film at the nanogram level based on micro-FTIR spectroscopy. Cellulose 2019, 26, 7175–7183. [Google Scholar] [CrossRef]
- Brar, V.; Kaur, G. Preparation and Characterization of Polyelectrolyte Complexes of Hibiscus esculentus (Okra) Gum and Chitosan. Int. J. Biomater. 2018, 2018, 4856287. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.C.; Cheng, C.H.; Ho, Y.C.; Tsai, M.L.; Mi, F.L. Active gellan gum/purple sweet potato composite films capable of monitoring pH variations. Food Hydrocoll. 2017, 69, 491–502. [Google Scholar] [CrossRef]
- Bakkari, M.E.; Bindiganavile, V.; Goncalves, J.; Boluk, Y. Preparation of cellulose nanofibers by TEMPO-oxidation of bleached chemi-thermomechanical pulp for cement applications. Carbohydr. Polym. 2019, 203, 238–245. [Google Scholar] [CrossRef]
- Martins, C.S.; Morgado, D.L.; Assis, O.B.G. Cashew gum-chitosan blended films: Spectral, mechanical and surface wetting evaluations. Macromol. Res. 2016, 24, 691–697. [Google Scholar] [CrossRef]
- Fan, B.; Yao, Q.; Wang, C.; Jin, C.; Wang, H.; Xiong, Y.; Li, S.; Sun, Q. Natural cellulose nanofiber extracted from cell wall of bamboo leaf and its derived multifunctional aerogel. Polym. Compos. 2018, 39, 3869–3876. [Google Scholar] [CrossRef]
- Li, J.; Hu, X.; Li, X.; Ma, Z. Effects of acetylation on the emulsifying properties of Artemisia sphaerocephala Krasch. polysaccharide. Carbohydr. Polym. 2016, 144, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Moloney, M.; Robbins, R.J.; Collins, T.M.; Kondo, T.; Yoshida, K.; Dangles, O. Red cabbage anthocyanins: The influence of d-glucose acylation by hydroxycinnamic acids on their structural transformations in acidic to mildly alkaline conditions and on the resulting color. Dyes Pigments 2018, 158, 342–352. [Google Scholar] [CrossRef]
- Prietto, L.; Mirapalhete, T.C.; Pinto, V.Z.; Hoffmann, J.F.; Vanier, N.L.; Lim, L.T.; Guerra Dias, A.R.; da Rosa Zavareze, E. pH-sensitive films containing anthocyanins extracted from black bean seed coat and red cabbage. LWT Food Sci. Technol. 2017, 80, 492–500. [Google Scholar] [CrossRef]
- Zhai, X.; Shi, J.; Zou, X.; Wang, S.; Jiang, C.; Zhang, J.; Huang, X.; Zhang, W.; Holmes, M. Novel colorimetric films based on starch/polyvinyl alcohol incorporated with roselle anthocyanins for fish freshness monitoring. Food Hydrocoll. 2017, 69, 308–317. [Google Scholar] [CrossRef] [Green Version]















| Film-Forming Solution | η0 (Pa·s) | K (s) | p | R2 |
|---|---|---|---|---|
| AFR0 | 1.3857 ± 0.8004 | 2.1880 ± 0.1963 | 0.7639 ± 0.0315 | 0.9994 |
| AFR5 | 1.3538 ± 0.3542 | 2.1631 ± 0.0974 | 0.7236 ± 0.0138 | 0.9999 |
| AFR10 | 1.3250 ± 0.4616 | 1.9931 ± 0.1225 | 0.7348 ± 0.0206 | 0.9997 |
| AFR15 | 1.3015 ± 0.3603 | 1.8871 ± 0.0955 | 0.7350 ± 0.0175 | 0.9998 |
| Sample | pH | L* | a* | b* | ΔE | Before | After |
|---|---|---|---|---|---|---|---|
| AFR5 | 3.0 | 79.91 ± 0.36 a | 6.13 ± 0.40 f | −2.98 ± 0.39 a | 6.18 ± 0.31 d |  |  |
| 4.0 | 81.31 ± 0.75 b | 2.86 ± 0.08 e | −3.47 ± 0.86 a | 4.84 ± 0.40 cd |  |  | |
| 5.0 | 82.01 ± 0.47 bc | 2.24 ± 0.03 d | −3.80 ± 0.73 a | 4.92 ± 1.38 cd |  |  | |
| 6.0 | 81.81 ± 0.43 b | 2.02 ± 0.02 d | −3.31 ± 0.73 a | 4.23 ± 0.23 bc |  |  | |
| 7.0 | 81.00 ± 0.86 ab | 1.24 ± 0.03 c | −1.19 ± 1.37 b | 2.82 ± 1.70 ab |  |  | |
| 8.0 | 83.12 ± 0.36 c | 1.33 ± 0.05 c | −4.43 ± 0.35 a | 4.61 ± 0.55 cd |  |  | |
| 9.0 | 82.05 ± 0.58 bc | −0.48 ± 0.30 b | −0.87 ± 1.12 c | 2.37 ± 0.53 a |  |  | |
| 10.0 | 79.90 ± 0.97 a | −4.08 ± 0.39 a | −0.72 ± 1.09 c | 6.12 ± 0.37 d |  |  | |
| AFR10 | 3.0 | 78.71 ± 0.20 b | 5.86 ± 0.33 f | 0.48 ± 0.49 b | 10.46 ± 1.02 bc |  |  |
| 4.0 | 78.08 ± 1.01 b | 3.15 ± 0.04 e | 2.62 ± 1.04 bc | 8.59 ± 0.62 abc |  |  | |
| 5.0 | 77.92 ± 0.22 b | 2.69 ± 0.18 de | 3.55 ± 0.58 cd | 8.871 ± 0.77 abc |  |  | |
| 6.0 | 77.20 ± 0.37 b | 2.44 ± 0.07 cde | 3.94 ± 0.24 cd | 8.54 ± 0.51 abc |  |  | |
| 7.0 | 77.89 ± 1.52 b | 1.93 ± 0.20 bc | 4.25 ± 2.86 cd | 7.64 ± 3.35 a |  |  | |
| 8.0 | 81.31 ± 0.77 c | 1.98 ± 0.06 bcd | −2.39 ± 1.14 a | 11.22 ± 1.55 c |  |  | |
| 9.0 | 77.48 ± 0.60 b | 1.66 ± 0.22 b | 4.50 ± 0.37 cd | 7.10 ± 0.21 a |  |  | |
| 10.0 | 73.76 ± 0.76 a | −2.83 ± 1.01 a | 5.36 ± 1.94 d | 8.16 ± 0.68 ab |  |  | |
| AFR15 | 3.0 | 77.82 ± 0.77 c | 6.36 ± 0.46 e | 3.24 ± 0.97b | 12.48 ± 0.83 ab |  |  |
| 4.0 | 78.27 ± 2.53 cd | 3.93 ± 0.78 d | 4.08 ± 4.68 b | 12.14 ± 4.49 ab |  |  | |
| 5.0 | 77.51 ± 0.76 bc | 3.08 ± 0.27 cd | 6.33 ± 1.04 bc | 10.37 ± 1.28 ab |  |  | |
| 6.0 | 79.20 ± 0.31 cd | 2.57 ± 0.08 bc | 2.83 ± 0.54 b | 14.05 ± 1.03 bc |  |  | |
| 7.0 | 75.48 ± 0.85 b | 2.51 ± 0.14 bc | 8.94 ± 1.10 c | 10.14 ± 1.08 a |  |  | |
| 8.0 | 80.38 ± 0.10 d | 2.25 ± 0.01 bc | −0.92 ± 0.61 a | 16.53 ± 0.29 c |  |  | |
| 9.0 | 78.05 ± 1.04 c | 1.80 ± 0.19 b | 3.70 ± 1.78 b | 12.02 ± 2.33 ab |  |  | |
| 10.0 | 72.55 ± 1.28 a | −1.95 ± 1.09 a | 5.83 ± 2.33 bc | 11.45 ± 1.34 ab |  |  |
| Sample | Humidity | L* | a* | b* | ΔE | Before | After |
|---|---|---|---|---|---|---|---|
| AFR5 | 33% | 77.73 ±0.39 a | −8.47 ± 0.35 a | 17.57 ± 1.08 c | 20.29 ± 1.47 c |  |  |
| 75% | 78.92 ± 0.08 b | −6.09 ± 0.12 b | 11.87 ± 0.22 b | 14.82 ± 0.19 b |  |  | |
| 90% | 79.34 ± 0.25 b | −0.11 ± 0.07 c | 2.67 ± 0.32 a | 4.66 ± 0.18 a |  |  | |
| AFR10 | 33% | 70.66 ± 0.44 a | −7.22 ± 0.03 a | 31.95 ± 0.81 c | 25.02 ± 1.10 c |  |  |
| 75% | 72.21 ± 0.38 b | −5.51 ± 0.11 b | 25.41 ± 1.19 b | 19.22 ± 1.69 b |  |  | |
| 90% | 73.97 ± 0.55 c | −2.25 ± 0.38 c | 15.32 ± 1.24 a | 10.89 ± 1.10 a |  |  | |
| AFR15 | 33% | 69.45 ± 0.31 a | −4.92 ± 0.22 a | 31.48 ± 0.73 c | 20.96 ± 1.00 c |  |  |
| 75% | 69.48 ± 0.32 a | −3.51 ± 0.28 b | 28.20 ± 0.64 b | 17.09 ± 0.75 b |  |  | |
| 90% | 68.73 ± 0.47 a | −0.70 ± 0.11 c | 24.21 ± 0.93 a | 12.29 ± 0.51 a |  |  |
| Storage Time (h) | L* | a* | b* | ΔE | Photograph |
|---|---|---|---|---|---|
| 0 | 75.09 ± 0.58 ab | 0.32 ± 0.03 c | −0.31 ± 0.52 a | — |  |
| 12 | 75.78 ± 0.99 b | −4.18 ± 0.19 b | 7.61 ± 0.12 b | 9.22 ± 0.05 b |  |
| 24 | 73.75 ± 0.65 a | −5.05 ± 0.21 a | 10.35 ± 0.41 c | 12.04 ± 0.83 c |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, T.; Wang, L. Preparation, Characterization and Application of a Low Water-Sensitive Artemisia sphaerocephala Krasch. Gum Intelligent Film Incorporated with Anionic Cellulose Nanofiber as a Reinforcing Component. Polymers 2020, 12, 247. https://doi.org/10.3390/polym12010247
Liang T, Wang L. Preparation, Characterization and Application of a Low Water-Sensitive Artemisia sphaerocephala Krasch. Gum Intelligent Film Incorporated with Anionic Cellulose Nanofiber as a Reinforcing Component. Polymers. 2020; 12(1):247. https://doi.org/10.3390/polym12010247
Chicago/Turabian StyleLiang, Tieqiang, and Lijuan Wang. 2020. "Preparation, Characterization and Application of a Low Water-Sensitive Artemisia sphaerocephala Krasch. Gum Intelligent Film Incorporated with Anionic Cellulose Nanofiber as a Reinforcing Component" Polymers 12, no. 1: 247. https://doi.org/10.3390/polym12010247
APA StyleLiang, T., & Wang, L. (2020). Preparation, Characterization and Application of a Low Water-Sensitive Artemisia sphaerocephala Krasch. Gum Intelligent Film Incorporated with Anionic Cellulose Nanofiber as a Reinforcing Component. Polymers, 12(1), 247. https://doi.org/10.3390/polym12010247