Triple Layer Tungsten Trioxide, Graphene, and Polyaniline Composite Films for Combined Energy Storage and Electrochromic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thin Film Electrode Preparation
2.3. Characterizations
3. Results and Discussion
3.1. Thin Film Electrode Preparation
3.2. Materials Characterization
3.3. Electrochromic Behaviors
3.4. Capacitive Energy Storage Performances
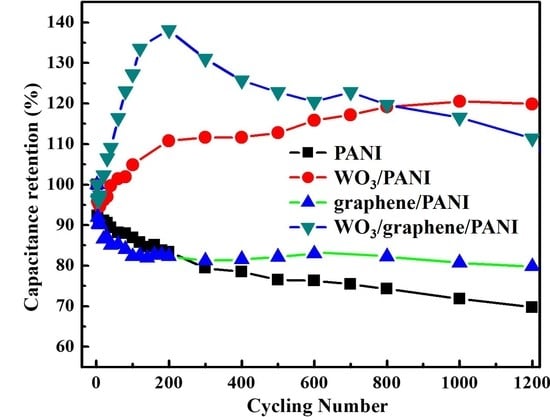
3.5. Cycling Stability
4. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Lyu, H.; Li, Y.; Jafta, C.J.; Bridges, C.A.; Meyer, H.M., III; Borisevich, A.; Paranthaman, M.P.; Dai, S.; Sun, X.-G. Bis (trimethylsilyl) 2-fluoromalonate derivatives as electrolyte additives for high voltage lithium ion batteries. J. Power Sources 2019, 412, 527–535. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Xie, H.; Guo, J.; Lyu, H.; Li, Y.; Sun, Z.; Wang, H.; Guo, Z. Electrospun titania nanofibers segregated by graphene oxide for improved visible light photocatalysis. Appl. Catal. B Environ. 2017, 201, 470–478. [Google Scholar] [CrossRef]
- Liu, J.; Guo, S.; Hu, C.; Lyu, H.; Yan, X.; Guo, Z. Advanced Nanocomposite Electrodes for Lithium-Ion Batteries. Multifunct. Nanocomposites Energy Environ. Appl. 2018, 1, 7–32. [Google Scholar]
- Lyu, H.; Liu, J.; Qiu, S.; Cao, Y.; Hu, C.; Guo, S.; Guo, Z. Carbon composite spun fibers with in situ formed multicomponent nanoparticles for a lithium-ion battery anode with enhanced performance. J. Mater. Chem. A 2016, 4, 9881–9889. [Google Scholar] [CrossRef]
- Hu, C.; Qiu, S.; Lu, G.; Cao, H.; Lv, H.; Guo, S.; Liu, J. Enhanced electrochemical performance of barium hexaferrite nanoplates by Zn 2+ doping serving as anode materials. Rsc. Adv. 2015, 5, 70749–70757. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [Green Version]
- Lyu, H.; Jafta, C.J.; Popovs, I.; Meyer, H.M.; Hachtel, J.A.; Huang, J.; Sumpter, B.G.; Dai, S.; Sun, X.-G. A dicyanobenzoquinone based cathode material for rechargeable lithium and sodium ion batteries. J. Mater. Chem. A 2019, 7, 17888–17895. [Google Scholar] [CrossRef]
- Kung, C.-W.; Wang, T.C.; Mondloch, J.E.; Fairen-Jimenez, D.; Gardner, D.M.; Bury, W.; Klingsporn, J.M.; Barnes, J.C.; van Duyne, R.; Stoddart, J.F.; et al. Metal–organic framework thin films composed of free-standing acicular nanorods exhibiting reversible electrochromism. Chem. Mater. 2013, 25, 5012–5017. [Google Scholar] [CrossRef]
- Monk, P.; Mortimer, R.; Rosseinsky, D. Electrochromism and Electrochromic Devices; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Scherer, M.R.J.; Li, L.; Cunha, P.M.S.; Scherman, O.A.; Steiner, U. Enhanced Electrochromism in Gyroid-Structured Vanadium Pentoxide. Adv. Mater. 2012, 24, 1217–1221. [Google Scholar] [CrossRef]
- Heuer, H.W.; Wehrmann, R.; Kirchmeyer, S. Electrochromic Window Based on Conducting Poly (3, 4-ethylenedioxythiophene)–Poly (styrene sulfonate). Adv. Funct. Mater. 2002, 12, 89–94. [Google Scholar] [CrossRef]
- Wu, N.; Lv, H.; Liu, J.; Liu, Y.; Wang, S.; Liu, W. Improved electromagnetic wave absorption of Co nanoparticles decorated carbon nanotubes derived from synergistic magnetic and dielectric losses. Phys. Chem. Chem. Phys. 2016, 18, 31542–31550. [Google Scholar] [CrossRef]
- Qiu, S.; Lyu, H.; Liu, J.; Liu, Y.; Wu, N.; Liu, W. Facile synthesis of porous nickel/carbon composite microspheres with enhanced electromagnetic wave absorption by magnetic and dielectric losses. Acs Appl. Mater. Interfaces 2016, 8, 20258–20266. [Google Scholar] [CrossRef]
- Lin, F.; Nordlund, D.; Weng, T.-C.; Sokaras, D.; Jones, K.M.; Reed, R.B.; Gillaspie, D.T.; Weir, D.G.J.; Moore, R.G.; Dillon, A.C.; et al. Origin of electrochromism in high-performing nanocomposite nickel oxide. ACS Appl. Mater. Interfaces 2013, 5, 3643–3649. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Reynolds, J.R. Color control in π-conjugated organic polymers for use in electrochromic devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef]
- Thakur, V.K.; Ding, G.; Ma, J.; Lee, P.S.; Lu, X. Hybrid materials and polymer electrolytes for electrochromic device applications. Adv. Mater. 2012, 24, 4071–4096. [Google Scholar] [CrossRef]
- Hu, C.; Cao, H.; Wang, S.; Wu, N.; Qiu, S.; Lyu, H.; Liu, J. Synthesis of strontium hexaferrite nanoplates and the enhancement of their electrochemical performance by Zn 2+ doping for high-rate and long-life lithium-ion batteries. New J. Chem. 2017, 41, 6427–6435. [Google Scholar] [CrossRef]
- Wang, K.; Wu, H.; Meng, Y.; Zhang, Y.; Wei, Z. Integrated energy storage and electrochromic function in one flexible device: An energy storage smart window. Energy Environ. Sci. 2012, 5, 8384–8389. [Google Scholar] [CrossRef]
- Olinga, T.E.; Fraysse, J.; Travers, J.P.; Dufresne, A.; Pron, A. Highly conducting and solution-processable polyaniline obtained via protonation with a new sulfonic acid containing plasticizing functional groups. Macromolecules 2000, 33, 2107–2113. [Google Scholar] [CrossRef]
- Lyu, H.; Liu, J.; Mahurin, S.; Dai, S.; Guo, Z.; Sun, X.-G. Polythiophene coated aromatic polyimide enabled ultrafast and sustainable lithium ion batteries. J. Mater. Chem. A 2017, 5, 24083–24092. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Bhadra, S.; Singha, N.K.; Khastgir, D. Electrochemical synthesis of polyaniline and its comparison with chemically synthesized polyaniline. J. Appl. Polym. Sci. 2007, 104, 1900–1904. [Google Scholar] [CrossRef]
- Jang, J.; Oh, J.H. Fabrication of a highly transparent conductive thin film from polypyrrole/poly (methyl methacrylate) core/shell nanospheres. Adv. Funct. Mater. 2005, 15, 494–502. [Google Scholar] [CrossRef]
- Unur, E.; Jung, J.-H.; Mortimer, R.J.; Reynolds, J.R. Dual-polymer electrochromic film characterization using bipotentiostatic control. Chem. Mater. 2008, 20, 2328–2334. [Google Scholar] [CrossRef]
- Matsui, J.; Kikuchi, R.; Miyashita, T. A trilayer film approach to multicolor electrochromism. J. Am. Chem. Soc. 2014, 136, 842–845. [Google Scholar] [CrossRef]
- Tseng, C.-Y.; Hu, C.-W.; Huang, K.-C.; Chang, L.-C.; Vittal, R.; Ho, K.-C. Ion transport across the film of poly(5,6-dimethoxyindole-2-carboxylic acid) in relation to its electrochromic switching: An electrochemical quartz crystal microbalance study. Electrochim. Acta 2013, 101, 232–237. [Google Scholar] [CrossRef]
- Yoon, S.; Kang, E.; Kim, J.K.; Lee, C.W.; Lee, J. Development of high-performance supercapacitor electrodes using novel ordered mesoporous tungsten oxide materials with high electrical conductivity. Chem. Commun. 2011, 47, 1021–1023. [Google Scholar] [CrossRef] [Green Version]
- Jo, C.; Hwang, J.; Song, H.; Dao, A.H.; Kim, Y.-T.; Lee, S.H.; Hong, S.W.; Yoon, S.; Lee, J. Block-Copolymer-Assisted One-Pot Synthesis of Ordered Mesoporous WO3−x/Carbon Nanocomposites as High-Rate-Performance Electrodes for Pseudocapacitors. Adv. Funct. Mater. 2013, 23, 3747–3754. [Google Scholar] [CrossRef]
- Fu, Y.; Gu, H.; Yan, X.; Liu, J.; Wang, Y.; Huang, J.; Li, X.; Lv, H.; Wang, X.; Guo, J.; et al. Chromium (III) oxide carbon nanocomposites lithium-ion battery anodes with enhanced energy conversion performance. Chem. Eng. J. 2015, 277, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Wen, R.-T.; Niklasson, G.A.; Granqvist, C.G. Sustainable Rejuvenation of Electrochromic WO3 Films. Acs Appl. Mater. Interfaces 2015, 7, 28100–28104. [Google Scholar] [CrossRef] [Green Version]
- Shen, K.; Ran, F.; Tan, Y.; Niu, X.; Fan, H.; Yan, K.; Kong, L.; Kang, L. Toward interconnected hierarchical porous structure via chemical depositing organic nano-polyaniline on inorganic carbon scaffold for supercapacitor. Synth. Met. 2015, 199, 205–213. [Google Scholar] [CrossRef]
- Jeon, J.-W.; Kwon, S.R.; Li, F.; Lutkenhaus, J.L. Spray-on polyaniline/poly (acrylic acid) electrodes with enhanced electrochemical stability. Acs Appl. Mater. Interfaces 2015, 7, 24150–24158. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wei, T.; Fan, Z.; Qian, W.; Zhang, M.; Shen, X.; Wei, F. Preparation of graphene nanosheet/carbon nanotube/polyaniline composite as electrode material for supercapacitors. J. Power Sources 2010, 195, 3041–3045. [Google Scholar] [CrossRef]
- Lv, H.; Qiu, S.; Lu, G.; Fu, Y.; Li, X.; Hu, C.; Liu, J. Nanostructured antimony/carbon composite fibers as anode material for lithium-ion battery. Electrochim. Acta 2015, 151, 214–221. [Google Scholar] [CrossRef]
- Li, X.; Gu, H.; Liu, J.; Wei, H.; Qiu, S.; Fu, Y.; Lv, H.; Lu, G.; Wang, Y.; Guo, Z. Multi-walled carbon nanotubes composited with nanomagnetite for anodes in lithium ion batteries. RSC Adv. 2015, 5, 7237–7244. [Google Scholar] [CrossRef]
- Lu, G.; Qiu, S.; Lv, H.; Fu, Y.; Liu, J.; Li, X.; Bai, Y.-J. Li-ion storage performance of MnO nanoparticles coated with nitrogen-doped carbon derived from different carbon sources. Electrochim. Acta 2014, 146, 249–256. [Google Scholar] [CrossRef]
- Kumar, N.A.; Baek, J.-B. Electrochemical supercapacitors from conducting polyaniline–graphene platforms. Chem. Commun. 2014, 50, 6298–6308. [Google Scholar] [CrossRef]
- Thapaliya, B.P.; Do-Thanh, C.L.; Jafta, C.J.; Tao, R.; Lyu, H.; Borisevich, A.Y.; Yang, S.Z.; Sun, X.G.; Dai, S. Simultaneously Boosting the Ionic Conductivity and Mechanical Strength of Polymer Gel Electrolyte Membranes by Confining Ionic Liquids into Hollow Silica Nanocavities. Batter. Supercaps 2019. [Google Scholar] [CrossRef]
- Qiu, S.; Lu, G.; Liu, J.; Lyu, H.; Hu, C.; Li, B.; Yan, X.; Guo, J.; Guo, Z. Enhanced electrochemical performances of MoO 2 nanoparticles composited with carbon nanotubes for lithium-ion battery anodes. RSC Adv. 2015, 5, 87286–87294. [Google Scholar] [CrossRef]
- Lyu, H.; Li, P.; Liu, J.; Mahurin, S.; Chen, J.; Hensley, D.K.; Veith, G.M.; Guo, Z.; Dai, S.; Sun, X.G. Aromatic Polyimide/Graphene Composite Organic Cathodes for Fast and Sustainable Lithium-Ion Batteries. ChemSusChem 2018, 11, 763–772. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Lei, S.; Song, Y. Graphene-based polyaniline nanocomposites: Preparation, properties and applications. J. Mater. Chem. A 2014, 2, 4491–4509. [Google Scholar] [CrossRef]
- Tong, Y.; Lyu, H.; Xu, Y.; Thapaliya, B.P.; Li, P.; Sun, X.-G.; Dai, S. All-solid-state interpenetrating network polymer electrolytes for long cycle life of lithium metal batteries. J. Mater. Chem. A 2018, 6, 14847–14855. [Google Scholar] [CrossRef]
- Thapaliya, B.P.; Jafta, C.J.; Lyu, H.; Xia, J.; Meyer, H.M., III; Paranthaman, M.P.; Sun, X.G.; Bridges, C.A.; Dai, S. Fluorination of MXene by Elemental F2 as Electrode Material for Lithium-Ion Batteries. ChemSusChem 2019, 12, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Pruneanu, S.; Veress, E.; Marian, I.; Oniciu, L. Characterization of polyaniline by cyclic voltammetry and UV-Vis absorption spectroscopy. J. Mater. Sci. 1999, 34, 2733–2739. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, J.P.; Zhang, D.; Qiao, Y.Q.; Xia, X.H.; Wang, X.L.; Gu, C.D. Multicolor electrochromic polyaniline-WO3 hybrid thin films: One-pot molecular assembling synthesis. J. Mater. Chem. 2011, 21, 17316–17324. [Google Scholar] [CrossRef]
- Huang, W.S.; MacDiarmid, A.G. Optical properties of polyaniline. Polymer 1993, 34, 1833–1845. [Google Scholar] [CrossRef]










| Film | CV CS. 50 mV/s (mF cm−2) | GCD Cs. 0.08 mA/cm2 (mF cm−2) | Energy Density (mWh/m2) | Power Density (mW/m2) |
|---|---|---|---|---|
| WO3 | 0.489 | 0.253 | 0.688 | 1.770 |
| WO3/graphene | 0.764 | 2.586 | 6.360 | 1.683 |
| PANI | 1.371 | 3.693 | 19.69 | 2.478 |
| WO3/PANI | 1.856 | 4.312 | 30.95 | 2.879 |
| graphene/PANI | 2.629 | 8.709 | 63.29 | 2.895 |
| WO3/graphene/PANI | 3.414 | 11.267 | 88.17 | 3.003 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, H. Triple Layer Tungsten Trioxide, Graphene, and Polyaniline Composite Films for Combined Energy Storage and Electrochromic Applications. Polymers 2020, 12, 49. https://doi.org/10.3390/polym12010049
Lyu H. Triple Layer Tungsten Trioxide, Graphene, and Polyaniline Composite Films for Combined Energy Storage and Electrochromic Applications. Polymers. 2020; 12(1):49. https://doi.org/10.3390/polym12010049
Chicago/Turabian StyleLyu, Hailong. 2020. "Triple Layer Tungsten Trioxide, Graphene, and Polyaniline Composite Films for Combined Energy Storage and Electrochromic Applications" Polymers 12, no. 1: 49. https://doi.org/10.3390/polym12010049
APA StyleLyu, H. (2020). Triple Layer Tungsten Trioxide, Graphene, and Polyaniline Composite Films for Combined Energy Storage and Electrochromic Applications. Polymers, 12(1), 49. https://doi.org/10.3390/polym12010049