Porcine Collagen–Bone Composite Induced Osteoblast Differentiation and Bone Regeneration In Vitro and In Vivo
Abstract
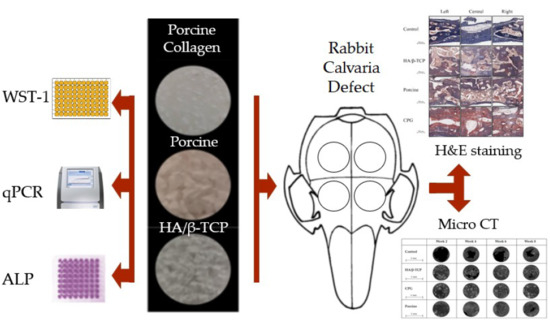
:1. Introduction
2. Materials and Methods
2.1. Graft Materials
2.2. Cell Culture and Seeding
2.3. Cell Cytotoxicity
2.4. Assessment of Cell Morphology by Fluorescence Microscopy
2.5. Alkaline Phosphatase Activity
2.6. Real-Time Polymerase Chain Reaction (qPCR)
2.7. In Vivo Test
2.8. Micro-CT Scanning Cortical Defect Closure
2.9. Histological Analysis
2.10. Statistical Analysis
3. Results
3.1. Cell Culture and Organization
3.2. Alkaline Phosphatase Assay
3.3. Real-Time Polymerase Chain Reaction (qPCR)
3.4. Defect Closure
3.5. Histological and Histomorphometric Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Van der Stok, J.; Van Lieshout, E.M.; El-Massoudi, Y.; Van Kralingen, G.H.; Patka, P. Bone substitutes in the Netherlands—A systematic literature review. Acta Biomater. 2011, 7, 739–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Yeung, K.W. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Finkemeier, C.G. Bone-grafting and bone-graft substitutes. JBJS 2002, 84, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Faour, O.; Dimitriou, R.; Cousins, C.A.; Giannoudis, P.V. The use of bone graft substitutes in large cancellous voids: Any specific needs? Injury 2011, 42, S87–S90. [Google Scholar] [CrossRef]
- Blank, A.T.; Riesgo, A.M.; Gitelis, S.; Rapp, T.B. Bone grafts, substitutes, and augments in benign orthopaedic conditions: Current concepts. Bull. NYU Hosp. Jt. Dis. 2017, 75, 119. [Google Scholar]
- Salamanca, E.; Hsu, C.C.; Huang, H.M.; Teng, N.C.; Lin, C.T.; Pan, Y.H.; Chang, W.J. Bone regeneration using a porcine bone substitute collagen composite in vitro and in vivo. Sci. Rep. 2018, 8, 984. [Google Scholar] [CrossRef] [Green Version]
- Murugan, R.; Rao, K.P.; Kumar, T.S. Heat-deproteinated xenogeneic bone from slaughterhouse waste: Physico-chemical properties. Bull. Mater. Sci. 2003, 26, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Etok, S.E.; Valsami-Jones, E.; Wess, T.J.; Hiller, J.C.; Maxwell, C.A.; Rogers, K.D.; Manning, D.A.; White, M.L.; Lopez-Capel, E.; Collins, M.J.; et al. Structural and chemical changes of thermally treated bone apatite. J. Mater. Sci. 2007, 42, 9807–9816. [Google Scholar] [CrossRef]
- Cassetta, M.; Perrotti, V.; Calasso, S.; Piattelli, A.; Sinjari, B.; Iezzi, G. Bone formation in sinus augmentation procedures using autologous bone, porcine bone, and a 50: 50 mixture: A human clinical and histological evaluation at 2 months. Clin. Oral Implant. Res. 2015, 26, 1180–1184. [Google Scholar] [CrossRef]
- Sandor, G.; Lindholm, T.; Clokie, C. Bone regeneration of the cranio-maxillofacial and dento-alveolar skeletons in the framework of tissue engineering. Top. Tissue Eng. 2003, 7, 1–46. [Google Scholar]
- Figueiredo, M.; Henriques, J.; Martins, G.; Guerra, F.; Judas, F.; Figueiredo, H. Physicochemical characterization of biomaterials commonly used in dentistry as bone substitutes—comparison with human bone. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 92, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Capparé, P.; Romanos, G.E.; Mariani, E.; Benasciutti, E.; Gherlone, E. Corticocancellous porcine bone in the healing of human extraction sockets: Combining histomorphometry with osteoblast gene expression profiles in vivo. Int. J. Oral Maxillofac. Implant. 2011, 26, 866–872. [Google Scholar]
- Schwarz, F.; Rothamel, D.; Herten, M.; Sager, M.; Becker, J. Angiogenesis pattern of native and cross-linked collagen membranes: An immunohistochemical study in the rat. Clin. Oral Implant. Res. 2006, 17, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, E.; Tsai, C.Y.; Pan, Y.H.; Lin, Y.T.; Huang, H.M.; Teng, N.C.; Lin, C.T.; Feng, S.W.; Chang, W.J. In vitro and in vivo study of a novel porcine collagen membrane for guided bone regeneration. Materials 2016, 9, 949. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.P.; Buckley, C.; Taylor, B.L.; Sahyoun, C.C.; Patel, S.D.; Mont, A.J.; Mai, L.; Patel, S. and Freeman, J.W. Mechanical and Biological Evaluation of a Hydroxyapatite-Reinforced Scaffold for Bone Regeneration. J. Biomed. Mater. Res. Part A 2019, 107, 732–741. [Google Scholar] [CrossRef]
- Pagliani, L.; Andersson, P.; Lanza, M.; Nappo, A.; Verrocchi, D.; Volpe, S.; Sennerby, L. A collagenated porcine bone substitute for augmentation at Neoss implant sites: A prospective 1-year multicenter case series study with histology. Clin. Implant Dent. Relat. Res. 2012, 14, 746–758. [Google Scholar] [CrossRef]
- Barone, A.; Toti, P.; Quaranta, A.; Alfonsi, F.; Cucchi, A.; Negri, B.; Di Felice, R.; Marchionni, S.; Calvo-Guirado, J.L.; Covani, U.; et al. Clinical and Histological changes after ridge preservation with two xenografts: Preliminary results from a multicentre randomized controlled clinical trial. J. Clin. Periodontol. 2017, 44, 204–214. [Google Scholar] [CrossRef]
- Barone, A.; Toti, P.; Menchini-Fabris, G.B.; Derchi, G.; Marconcini, S.; Covani, U. Extra oral digital scanning and imaging superimposition for volume analysis of bone remodeling after tooth extraction with and without 2 types of particulate porcine mineral insertion: A randomized controlled trial. Clin. Implant Dent. Relat. Res. 2017, 19, 750–759. [Google Scholar] [CrossRef]
- Salamanca, E.; Lee, W.F.; Lin, C.Y.; Huang, H.M.; Lin, C.T.; Feng, S.W.; Chang, W.J. A novel porcine graft for regeneration of bone defects. Materials 2015, 8, 2523–2536. [Google Scholar] [CrossRef] [Green Version]
- Le GUEHENNEC, L.; Goyenvalle, E.; Aguado, E.; Pilet, P.; D’Arc, M.B.; Bilban, M.; Spaethe, R.; Daculsi, G. MBCP® biphasic calcium phosphate granules and tissucol® fibrin sealant in rabbit femoral defects: The effect of fibrin on bone ingrowth. J. Mater. Sci. Mater. Med. 2005, 16, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Ngamwongsatit, P.; Banada, P.P.; Panbangred, W.; Bhunia, A.K. WST-1-based cell cytotoxicity assay as a substitute for MTT-based assay for rapid detection of toxigenic Bacillus species using CHO cell line. J. Microbiol. Methods 2008, 73, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.-K.; Jeon, J.-Y.; Kim, Y.-J.; Kim, S.-G.; Hwang, K.-G. Cell attachment and proliferation of osteoblast-like MG63 cells on silk fibroin membrane for guided bone regeneration. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sila-Asna, M.; Bunyaratvej, A.; Maeda, S.; Kitaguchi, H.; Bunyaratavej, N. Osteoblast differentiation and bone formation gene expression in strontium-inducing bone marrow mesenchymal stem cell. Kobe J. Med. Sci. 2007, 53, 25–35. [Google Scholar]
- Bimboim, H.; Doly, J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979, 7, 1513–1523. [Google Scholar] [CrossRef] [Green Version]
- Vogelstein, B.; Gillespie, D. Preparative and analytical purification of DNA from agarose. Proc. Natl. Acad. Sci. USA 1979, 76, 615–619. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sollazzo, V.; Palmieri, A.; Scapoli, L.; Martinelli, M.; Girardi, A.; Alviano, F.; Pellati, A.; Perrotti, V.; Carinci, F. Bio-Oss® acts on Stem cells derived from Peripheral Blood. Oman Med. J. 2010, 25, 26. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ahn, G.; Kim, C.; Lee, J.S.; Lee, I.G.; An, S.H.; Yun, W.S.; Kim, S.Y.; Shim, J.H. Synergistic Effects of Beta Tri-Calcium Phosphate and Porcine-Derived Decellularized Bone Extracellular Matrix in 3D-Printed Polycaprolactone Scaffold on Bone Regeneration. Macromol. Biosci. 2018, 18, 1800025. [Google Scholar] [CrossRef]
- Vincent, D.H.; Trivedi, M.K.; Branton, A.; Trivedi, D.; Nayak, G.; Mondal, S.C.; Jana, S. Influenced of Biofield Energy Healing Treatment on Vitamin D3 for the Assessment of Bone Health Parameters in MG-63 cells. viXra 2018. viXra:1807.0213. [Google Scholar]
- Ongaro, A.; Pellati, A.; Bagheri, L.; Rizzo, P.; Caliceti, C.; Massari, L.; De Mattei, M. Characterization of notch signaling during osteogenic differentiation in human osteosarcoma cell line MG63. J. Cell. Physiol. 2016, 231, 2652–2663. [Google Scholar] [CrossRef]
- Cappagli, V.; Potes, C.S.; Ferreira, L.B.; Tavares, C.; Eloy, C.; Elisei, R.; Sobrinho-Simões, M.; Wookey, P.J.; Soares, P. Calcitonin receptor expression in medullary thyroid carcinoma. PeerJ 2017, 5, 3778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyce, B.F.; Xing, L. The Rankl/Rank/Opg Pathway. Curr. Osteoporos. Rep. 2007, 5, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Ashley, J.W.; Ahn, J.; Hankenson, K.D. Notch signaling promotes osteoclast maturation and resorptive activity. J. Cell. Biochem. 2015, 116, 2598–2609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalcanti, S.C.S.X.B.; Pereira, C.L.; Mazzonetto, R.; de Moraes, M.; Moreira, R.W.F. Histological and histomorphometric analyses of calcium phosphate cement in rabbit calvaria. J. Cranio-Maxillofac. Surg. 2008, 36, 354–359. [Google Scholar] [CrossRef]
- Figueiredo, A.; Coimbra, P.; Cabrita, A.; Guerra, F.; Figueiredo, M. Comparison of a xenogeneic and an alloplastic material used in dental implants in terms of physico-chemical characteristics and in vivo inflammatory response. Mater. Sci. Eng. C 2013, 33, 3506–3513. [Google Scholar] [CrossRef] [Green Version]
- Nannmark, U.; Sennerby, L. The bone tissue responses to prehydrated and collagenated cortico-cancellous porcine bone grafts: A study in rabbit maxillary defects. Clin. Implant Dent. Relat. Res. 2008, 10, 264–270. [Google Scholar] [CrossRef]
- Scarano, A.; Lorusso, F.; Ravera, L.; Mortellaro, C.; Piattelli, A. Bone regeneration in iliac crestal defects: An experimental study on sheep. BioMed Res. Int. 2016, 2016, 4086870. [Google Scholar] [CrossRef] [Green Version]
- Covani, U.; Cornelini, R.; Barone, A. Buccal bone augmentation around immediate implants with and without flap elevation: A modified approach. Int. J. Oral Maxillofac. Implant. 2008, 23, 25. [Google Scholar]
- Barone, A.; Cornelini, R.; Ciaglia, R.; Covani, U. Implant placement in fresh extraction sockets and simultaneous osteotome sinus floor elevation: A case series. Int. J. Periodontics Restor. Dent. 2008, 28, 3. [Google Scholar]









| Gene Symbol | Forward primer sequence (5′ > 3′) | Reverse primer sequence (5′ > 3′) |
|---|---|---|
| ALP | AGCCTTCCTGAAAGAGGATTGG | GCCAGTACTTGGGGTCTTTCT |
| OC | TCCTTTGGGGTTTGGCCTAC | CCAGCCTCCAGCACTGTTTA |
| RANKL | ACTGGCCTCTCACCTTTTCTG | AGCCATCCACCATCGCTTTC |
| CR | TTGCTGCCCGCAATTTATGA | TGCTGGCAAGATACTCAGGT |
| OPG | CTGGAACCCCAGAGCGAAAT | GCCTCCTCACACAGGGTAAC |
| RANK | GAAGGTGGACTGGCTACCAC | TTTCCTTCCCCTCCCCAGAA |
| GAPDH | CCTCCTGTTCGACAGTCAGC | CCTAGCCTCCCGGGTTTCTC |
| Defect Upper Side | Defect Lower Side | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 Weeks | 4 Weeks | 6 Weeks | 8 Weeks | 2 Weeks | 4 Weeks | 6 Weeks | 8 Weeks | |
| Control | 6.25 ± 7.99 * | 57.95 ± 17.04 * | 56.98 ± 35.34 * | 72.19 ± 24.08 * | 14.67 ± 11.48 * | 55.22 ± 15.8 * | 49.28 ± 31.01 * | 64.63 ± 19.04 * |
| HA/β-TCP | 65.56 ± 20.66 | 84.22 ± 6.95 | 93.48 ± 13.31 | 97.19 ± 1.65 | 61.74 ± 7.99 | 78.73 ± 12.45 | 93.99 ± 12.18 | 97.73 ± 1.57 |
| CPG | 73.65 ± 12.77 | 99.17 ± 1.06 | 100 ± 0 | 99.42 ± 1.3 ¶ | 52.88 ± 17.95 | 98.84 ± 1.28 | 100 ± 0 | 99.49 ± 1.14 ¶ |
| Porcine | 63.32 ± 18.44 | 94.58 ± 10.31 | 95.59 ± 7.37 | 94.66 ± 5.3 | 65.02 ± 20.42 | 95.56 ± 8.57 | 95.98 ± 6.77 | 95.64 ± 3.91 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamanca, E.; Hsu, C.C.; Yao, W.L.; Choy, C.S.; Pan, Y.H.; Teng, N.-C.; Chang, W.-J. Porcine Collagen–Bone Composite Induced Osteoblast Differentiation and Bone Regeneration In Vitro and In Vivo. Polymers 2020, 12, 93. https://doi.org/10.3390/polym12010093
Salamanca E, Hsu CC, Yao WL, Choy CS, Pan YH, Teng N-C, Chang W-J. Porcine Collagen–Bone Composite Induced Osteoblast Differentiation and Bone Regeneration In Vitro and In Vivo. Polymers. 2020; 12(1):93. https://doi.org/10.3390/polym12010093
Chicago/Turabian StyleSalamanca, Eisner, Chia Chen Hsu, Wan Ling Yao, Cheuk Sing Choy, Yu Hwa Pan, Nai-Chia Teng, and Wei-Jen Chang. 2020. "Porcine Collagen–Bone Composite Induced Osteoblast Differentiation and Bone Regeneration In Vitro and In Vivo" Polymers 12, no. 1: 93. https://doi.org/10.3390/polym12010093
APA StyleSalamanca, E., Hsu, C. C., Yao, W. L., Choy, C. S., Pan, Y. H., Teng, N. -C., & Chang, W. -J. (2020). Porcine Collagen–Bone Composite Induced Osteoblast Differentiation and Bone Regeneration In Vitro and In Vivo. Polymers, 12(1), 93. https://doi.org/10.3390/polym12010093