Preparation of Ultrafine Fly Ash-Based Superhydrophobic Composite Coating and Its Application to Foam Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
3. Results and Discussions
3.1. Analysis of UFA
3.2. Hydrophobicity Analysis of the Superhydrophobic Coating
3.2.1. Influence of the UFAs Modified Using Different Silane Coupling Reagents on the Coating Hydrophobicity
3.2.2. Influence of the Amount of the HFDS Additive on the Coating Hydrophobicity and Analysis of the UFA Surface
3.2.3. Influence of the UFA Dosage on the Coating Hydrophobicity and Hydrophobicity Analyses
3.3. Theoretical Hydrophobicity Analysis of the Surface Coating
3.3.1. Theoretical Analysis of the Surface Hydrophobicity of the Smooth HFDS Coating
3.3.2. Theoretical Hydrophobicity Analysis of No. 5 Coating
3.4. Application of the Superhydrophobic Composite Coatings onto the Foam Concrete
3.4.1. Performance Analysis of the UFA-Based Waterproof Base Coating
3.4.2. Influence of the Superhydrophobic Composite Coating on the Foam Concrete
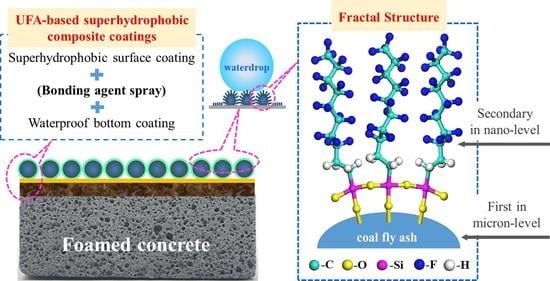
3.5. Construction Principle of the UFA-Based Superhydrophobic Composite Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Darmanin, T.; Guittard, F. Superhydrophobic and superoleophobic properties in nature. Mater. Today 2015, 18, 273–285. [Google Scholar] [CrossRef]
- Yin, W.; Zheng, Y.L.; Lu, H.; Zhang, X.J.; Tian, Y. Three-dimensional topographies of water surface dimples formed by superhydrophobic water strider legs. Appl. Phys. Lett. 2016, 109, 163701. [Google Scholar] [CrossRef]
- Gou, X.; Guo, Z. Superhydrophobic Plant Leaves with Micro-line Structures: An Optimal Biomimetic Objective in Bionic Engineering. J. Bionic Eng. 2018, 15, 851–858. [Google Scholar] [CrossRef]
- Husni, H.; Nazari, M.; Yee, H.; Rohim, R.; Yusuff, A.; Ariff, M.A.M.; Ahmad, N.; Leo, C.; Junaidi, M. Superhydrophobic rice husk ash coating on concrete. Constr. Build. Mater. 2017, 144, 385–391. [Google Scholar] [CrossRef]
- Song, H.; Cao, Z.; Xie, W.; Cheng, F.; Gasem, K.A.; Fan, M. Improvement of dispersion stability of filler based on fly ash by adding sodium hexametaphosphate in gas-sealing coating. J. Clean. Prod. 2019, 235, 259–271. [Google Scholar] [CrossRef]
- Yin, K.; Du, H.; Dong, X.; Wang, C.; Duan, J.A.; He, J. A simple way to achieve bioinspired hybrid wettability surface with micro/nanopatterns for efficient fog collection. Nanoscale 2017, 9, 14620–14626. [Google Scholar] [CrossRef]
- Li, Y.; Luong, D.X.; Zhang, J.; Tarkunde, Y.R.; Kittrell, C.; Sargunaraj, F.; Ji, Y.; Arnusch, C.J.; Tour, J.M. Laser-Induced Graphene in Controlled Atmospheres: From Superhydrophilic to Superhydrophobic Surfaces. Adv. Mater. 2017, 29, 1700496. [Google Scholar] [CrossRef]
- Liu, P.; Gao, Y.; Wang, F.; Yang, J.; Yu, X.; Zhang, W.; Yang, L. Superhydrophobic and self-cleaning behavior of Portland cement with lotus-leaf-like microstructure. J. Clean. Prod. 2017, 156, 775–785. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, C.H.; Cao, M.; Zhang, M.M.; Li, M.; Ma, J.Z. Highly transparent fluorine-free superhydrophobic silica nanotube coatings. Chem. Eng. J. 2017, 320, 244–252. [Google Scholar] [CrossRef]
- Farshchian, B.; Gatabi, J.R.; Bernick, S.M.; Park, S.; Lee, G.; Droopad, R.; Kim, N. Laser-induced superhydrophobic grid patterns on PDMS for droplet arrays formation. Appl. Surf. Sci. 2017, 396, 359–365. [Google Scholar] [CrossRef]
- Amigoni, S.; de Givenchy, E.T.; Dufay, M.; Guittard, F. Covalent Layer-by-Layer Assembled Superhydrophobic Organic−Inorganic Hybrid Films. Langmuir 2009, 25, 11073–11077. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Tri, P.; Tran, H.N.; Plamondon, C.O.; Tuduri, L.; Vo, D.V.N.; Nanda, S.; Mishra, A.; Chao, H.P.; Bajpai, A. Recent progress in the preparation, properties and applications of superhydrophobic nano-based coatings and surfaces: A review. Prog. Org. Coat. 2019, 132, 235–256. [Google Scholar] [CrossRef]
- Raj, A.; Sathyan, D.; Mini, K.M. Physical and functional characteristics of foam concrete: A review. Constr. Build. Mater. 2019, 221, 787–799. [Google Scholar] [CrossRef]
- Li, T.; Huang, F.; Zhu, J.; Tang, J.; Liu, J. Effect of foaming gas and cement type on the thermal conductivity of foamed concrete. Constr. Build. Mater. 2020, 231, 117197. [Google Scholar] [CrossRef]
- Khosravani, M.R.; Wagner, P.; Fröhlich, D.; Weinberg, K. Dynamic fracture investigations of ultra-high performance concrete by spalling tests. Eng. Struct. 2019, 201, 109844. [Google Scholar] [CrossRef]
- Weinberg, K.; Khosravani, M.R. On the tensile resistance of UHPC at impact. Eur. Phys. J. Spec. Top. 2018, 227, 167–177. [Google Scholar] [CrossRef]
- Liu, C.; Luo, J.; Li, Q.; Gao, S.; Jin, Z.; Li, S.; Zhang, P.; Chen, S. Water-resistance properties of high-belite sulphoaluminate cement-based ultra-light foamed concrete treated with different water repellents. Constr. Build. Mater. 2019, 228, 116798. [Google Scholar] [CrossRef]
- Amran, Y.M.; Farzadnia, N.; Ali, A.A. Properties and applications of foamed concrete; a review. Constr. Build. Mater. 2015, 101, 990–1005. [Google Scholar] [CrossRef]
- She, W.; Yang, J.; Hong, J.; Sun, D.; Mu, S.; Miao, C. Superhydrophobic concrete with enhanced mechanical robustness: Nanohybrid composites, strengthen mechanism and durability evaluation. Constr. Build. Mater. 2020, 247, 118563. [Google Scholar] [CrossRef]
- Kolkowitz, S.; Safira, A.; High, A.A.; Devlin, R.C.; Choi, S.; Unterreithmeier, Q.P.; Patterson, D.; Zibrov, A.; Manucharyan, V.E.; Park, H.; et al. Probing Johnson noise and ballistic transport in normal metals with a single-spin qubit. Science 2015, 347, 1129–1132. [Google Scholar] [CrossRef] [Green Version]
- Panesar, D.K.; Kanraj, D.; Abualrous, Y. Effect of transportation of fly ash: Life cycle assessment and life cycle cost analysis of concrete. Cem. Concr. Compos. 2019, 99, 214–224. [Google Scholar] [CrossRef]
- Cornelius, D.J.; Monroe, C.M. The unique properties of silicone and fluorosilicone elastomers. Polym. Eng. Sci. 1985, 25, 467–473. [Google Scholar] [CrossRef]
- Yang, H.T. Study on Fabrication of Functional Surface Materials with Different Wettability and Properties via Photopolymerization. Ph.D. Thesis, Beijing University of Chemical Technology, Beijing, China, 2017. [Google Scholar] [CrossRef]
- Nath, D.; Bandyopadhyay, S.; Gupta, S.; Yu, A.; Blackburn, D.; White, C. Surface-coated fly ash used as filler in biodegradable poly(vinyl alcohol) composite films: Part 1—The modification process. Appl. Surf. Sci. 2010, 256, 2759–2763. [Google Scholar] [CrossRef]
- Li, G.; Jiang, B.; Liu, H.; Ning, L.; Yi, D.; Wang, X.; Liu, Z. Superhydrophobic surface with lotus/petal effect and its improvement on fatigue resistance of heat-resistant steel. Prog. Org. Coat. 2019, 137, 105315. [Google Scholar] [CrossRef]
- Pan, Z.; Shahsavan, H.; Zhang, W.; Yang, F.K.; Zhao, B. Superhydro-oleophobic bio-inspired polydimethylsiloxane micropillared surface via FDTS coating/blending approaches. Appl. Surf. Sci. 2015, 324, 612–620. [Google Scholar] [CrossRef]
- Junaidi, M.U.M.; Azaman, S.H.; Ahmad, N.; Leo, C.; Lim, G.; Chan, D.; Yee, H. Superhydrophobic coating of silica with photoluminescence properties synthesized from rice husk ash. Prog. Org. Coat. 2017, 111, 29–37. [Google Scholar] [CrossRef]
- Yu, H.B.; Li, R.F. Preparation and properties of biomimetic superhydrophobic composite coating. Surf. Eng. 2016, 32, 79–84. [Google Scholar] [CrossRef]
- Kulinich, S.; Farzaneh, M. Hydrophobic properties of surfaces coated with fluoroalkylsiloxane and alkylsiloxane monolayers. Surf. Sci. 2004, 573, 379–390. [Google Scholar] [CrossRef]
- Herminghaus, S. Roughness-induced non-wetting. EPL Europhys. Lett. 2000, 52, 165–170. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, F.; Liu, P.; Zhang, W.; Yang, L. Superhydrophobic behavior of magnesium oxychloride cement surface with a dual-level fractal structure. Constr. Build. Mater. 2019, 210, 132–139. [Google Scholar] [CrossRef]
- Onda, T.; Shibuichi, S.; Satoh, N.; Tsujii, K. Super-Water-Repellent Fractal Surfaces. Langmuir 1996, 12, 2125–2127. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Marmur, A. Wetting on Hydrophobic Rough Surfaces: To be Heterogeneous or not to be? Langmuir 2003, 19, 8343–8348. [Google Scholar] [CrossRef]
- Cohen, N.; Dotan, A.; Dodiuk, H.; Kenig, S. Superhydrophobic Coatings and Their Durability. Mater. Manuf. Process. 2015, 31, 1143–1155. [Google Scholar] [CrossRef]
- Song, H.; Liu, J.; Xue, F.; Cheng, F. The application of ultra-fine fly ash in the seal coating for the wall of underground coal mine. Adv. Powder Technol. 2016, 27, 1645–1650. [Google Scholar] [CrossRef]
- Song, H.; Xie, W.; Liu, J.; Cheng, F.; Gasem, K.A.M.; Fan, M. Effect of surfactants on the properties of a gas-sealing coating modified with fly ash and cement. J. Mater. Sci. 2018, 53, 15142–15156. [Google Scholar] [CrossRef]
- Ellinas, K.; Tserepi, A.; Gogolides, E. Durable superhydrophobic and superamphiphobic polymeric surfaces and their applications: A review. Adv. Colloid Interface Sci. 2017, 250, 132–157. [Google Scholar] [CrossRef]
















| Composition | SiO2 | Al2O3 | Fe2O3 | CaO | SO3 | TiO2 | K2O | MgO | Others |
|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 50.1 | 34.0 | 4.8 | 3.7 | 1.1 | 1.0 | 1.0 | 0.7 | 3.8 |
| Sample Name | SEM Image and Contact Angle | AFM Image and Sq |
|---|---|---|
| Blank slide (No. 0 coating) |  |  |
| 1 g UFA (No. 1 coating) |  |  |
| 5 g UFA (No. 5 coating) |  |  |
| 10 g UFA (No. 10 coating) |  |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Tang, M.; Lei, X.; Feng, Z.; Cheng, F. Preparation of Ultrafine Fly Ash-Based Superhydrophobic Composite Coating and Its Application to Foam Concrete. Polymers 2020, 12, 2187. https://doi.org/10.3390/polym12102187
Song H, Tang M, Lei X, Feng Z, Cheng F. Preparation of Ultrafine Fly Ash-Based Superhydrophobic Composite Coating and Its Application to Foam Concrete. Polymers. 2020; 12(10):2187. https://doi.org/10.3390/polym12102187
Chicago/Turabian StyleSong, Huiping, Mingxiu Tang, Xu Lei, Zhengjun Feng, and Fangqin Cheng. 2020. "Preparation of Ultrafine Fly Ash-Based Superhydrophobic Composite Coating and Its Application to Foam Concrete" Polymers 12, no. 10: 2187. https://doi.org/10.3390/polym12102187
APA StyleSong, H., Tang, M., Lei, X., Feng, Z., & Cheng, F. (2020). Preparation of Ultrafine Fly Ash-Based Superhydrophobic Composite Coating and Its Application to Foam Concrete. Polymers, 12(10), 2187. https://doi.org/10.3390/polym12102187