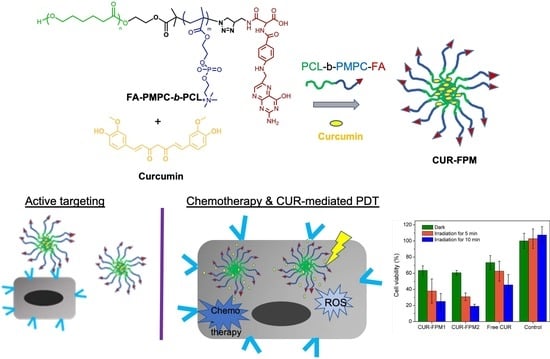
Folate-Targeted Curcumin-Encapsulated Micellar Nanosystem for Chemotherapy and Curcumin-Mediated Photodynamic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PMPC-b-PCL (PM) and N3-PMPC-b-PCL (N3-PM)
2.3. Synthesis of FA-PMPC-b-PCL (FPM)
2.4. General Characterization
2.5. Preparation of Blank and CUR-Loaded Micelles
2.6. Determination of Release Kinetics of CUR
2.7. Intracellular ROS Detection
2.8. Cell Culture and Cell Viability
2.9. In Vitro Phototoxicity Study
2.10. Subcellular Localization Study
2.11. Cellular Uptake Study
3. Results and Discussion
3.1. Preparation and Characterization of FPM
3.2. CUR Encapsulation and Aqueous Stability in Micelles
3.3. In Vitro Release Behavior
3.4. Intracellular ROS Generation Monitoring
3.5. In Vitro Cytotoxicity Evaluation
3.6. Subcellular Localization and Cellular Uptake
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [PubMed]
- Khayyal, M.T.; El-Hazek, R.M.; El-Sabbagh, W.A.; Frank, J.; Behnam, D.; Abdel-Tawab, M. Micellar solubilisation enhances the antiinflammatory activities of curcumin and boswellic acids in rats with adjuvant-induced arthritis. Nutrition 2018, 54, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.-Q.; Farha, A.K.; Kim, G.; Gul, K.; Gan, R.-Y.; Corke, H. Antimicrobial and anticancer applications and related mechanisms of curcumin-mediated photodynamic treatments. Trends Food Sci. Technol. 2020, 97, 341–354. [Google Scholar]
- Imran, M.; Ullah, A.; Saeed, F.; Nadeem, M.; Arshad, M.U.; Suleria, H.A.R. Cucurmin, anticancer, & antitumor perspectives: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1271–1293. [Google Scholar] [PubMed]
- Ruby, A.; Kuttan, G.; Dinesh Babu, K.; Rajasekharan, K.; Kuttan, R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995, 94, 79–83. [Google Scholar] [CrossRef]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer. 2011, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Beevers, C.S.; Huang, S. The targets of curcumin. Curr. Drug Targets. 2011, 12, 332–347. [Google Scholar] [CrossRef]
- Fadus, M.C.; Lau, C.; Bikhchandani, J.; Lynch, H.T. Curcumin: An age-old anti-inflammatory and anti-neoplastic agent. J. Tradit. Complement. Med. 2017, 7, 339–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Tao, Q.; Zou, Y.; Zhang, F.; Guo, M.; Wang, Y.; Wang, H.; Zhou, Q.; Yu, S. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: Characterizations and mechanisms. J. Agric. Food Chem. 2011, 59, 9280–9289. [Google Scholar] [CrossRef]
- Tsai, W.-H.; Yu, K.-H.; Huang, Y.-C.; Lee, C.-I. EGFR-targeted photodynamic therapy by curcumin-encapsulated chitosan/TPP nanoparticles. Int. J. Nanomedicine 2018, 13, 903–916. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Liu, Y.; Lin, L.; Zhao, Y.; Wang, X.; Zhong, M.; Xie, T.; Luo, Y.; Li, S.; Yang, R.; et al. Glycyrrhetinic acid modified and pH-sensitive mixed micelles improve the anticancer effect of curcumin in hepatoma carcinoma cells. RSC Adv. 2019, 9, 40131–40145. [Google Scholar] [CrossRef] [Green Version]
- Dagrada, G.; Rupel, K.; Zacchigna, S.; Tamborini, E.; Pilotti, S.; Cavalleri, A.; Fechner, L.E.; Laurini, E.; Smith, D.K.; Brich, S.; et al. Self-assembled nanomicelles as curcumin drug delivery vehicles: Impact on solitary fibrous tumor cell protein expression and viability. Mol. Pharm. 2018, 15, 4689–4701. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.C.; De Matos, R.P.A.; Primo, F.L.; Tedesco, A.C.; Rahal, P.; Calmon, M.F. Effect of curcumin-nanoemulsion associated with photodynamic therapy in breast adenocarcinoma cell line. Bioorg. Med. Chem. 2019, 27, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, K.; Chamani, E.; Hosseinzadeh, G.; Hosseinzadeh, R. Comparative study of photodynamic activity of methylene blue in the presence of salicylic acid and curcumin phenolic compounds on human breast cancer. Lasers Med. Sci. 2019, 34, 239–246. [Google Scholar] [CrossRef]
- Soleimani, H.; Amini, A.; Taheri, S.; Sajadi, E.; Shafikhani, S.; Schuger, L.A.; Reddy, V.B.; Ghoreishi, S.K.; Pouriran, R.; Chien, S.; et al. The effect of combined photobiomodulation and curcumin on skin wound healing in type I diabetes in rats. J. Photochem. Photobiol. B 2018, 181, 23–30. [Google Scholar] [CrossRef]
- Nowis, D.; Makowski, M.; Stokłosa, T.; Legat, M.; Issat, T.; Gołab̨, J. Direct tumor damage mechanisms of photodynamic therapy. Acta Biochim. Pol. 2005, 52, 339–352. [Google Scholar] [CrossRef] [Green Version]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer. 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Cai, M.; Cao, J.; Wu, Z.; Cheng, F.; Chen, Y.; Luo, X. In vitro and in vivo anti-tumor efficiency comparison of phosphorylcholine micelles with PEG micelles. Colloids Surf. B Biointerfaces 2017, 157, 268–279. [Google Scholar] [CrossRef]
- Kyomoto, M.; Moro, T.; Ishihara, K. UHMWPE Biomaterials Handbook: Ultra High Molecular Weight Polyethylene in Total Joint Replacement and Medical Devices, 3rd ed.; William Andrew: Oxford, UK, 2015. [Google Scholar]
- Wiltshire, J.T.; Qiao, G.G. Selectively degradable core cross-linked star polymers. Macromolecules 2006, 39, 9018–9027. [Google Scholar] [CrossRef]
- De, P.; Gondi, S.R.; Sumerlin, B.S. Folate-conjugated thermoresponsive block copolymers: Highly efficient conjugation and solution self-assembly. Biomacromolecules 2008, 9, 1064–1070. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Kim, T.H.; Wu, W.-C.; Huang, C.-M.; Wei, H.; Mount, C.W.; Tian, Y.; Jang, S.-H.; Pun, S.H.; Jen, A.K.-Y. pH-dependent, thermosensitive polymeric nanocarriers for drug delivery to solid tumors. Biomaterials 2013, 34, 4501–4509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, A.O.; Magnusson, J.P.; Moradi, E.; Soliman, M.; Wang, W.; Stolnik, S.; Thurecht, K.J.; Howdle, S.M.; Alexander, C. Modular construction of multifunctional bioresponsive cell-targeted nanoparticles for gene delivery. Bioconjugate Chem. 2011, 22, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.-Y.; Wang, T.-Y.; Liao, P.-W.; Wu, W.-C.; Chen, C.-Y. Folate-conjugated and dual stimuli-responsive mixed micelles loading indocyanine green for photothermal and photodynamic Therapy. Macromol. Biosci. 2018, 18, 1700409. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef] [Green Version]
- Vortherms, A.R.; Doyle, R.P.; Gao, D.; Debrah, O.; Sinko, P.J. Synthesis, characterization, and in vitro assay of folic acid conjugates of 3′-azido-3′-deoxythymidine (AZT): Toward targeted AZT based anticancer therapeutics. Nucleos. Nucleot. Nucleic Acids 2008, 27, 173–185. [Google Scholar] [CrossRef]
- Angarita, A.V.; Umaña-Perez, A.; Perez, L.D. Enhancing the performance of PEG-b-PCL-based nanocarriers for curcumin through its conjugation with lipophilic biomolecules. J. Bioact. Compat. Polym. 2020, 35, 399–413. [Google Scholar]
- Pillai, J.J.; Thulasidasan, A.K.T.; Anto, R.J.; Devika, N.C.; Ashwanikumara, N.; Vinod Kumar, G.S. Curcumin entrapped folic acid conjugated PLGA– PEG nanoparticles exhibit enhanced anticancer activity by site specific delivery. RSC Adv. 2015, 5, 25518–25524. [Google Scholar]
- Yang, C.; Chen, H.; Zhao, J.; Pang, X.; Xi, Y.; Zhai, G. Development of a folate-modified curcumin loaded micelle delivery system for cancer targeting. Colloids Surf. B Biointerfaces 2014, 121, 206–213. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, X.; Peng, S.; Chen, X.; Liu, W.; Liu, C. Novel folated pluronic F127 modified liposomes for delivery of curcumin: Preparation, release and cytotoxicity. J. Microencapsul. 2020, 37, 220–229. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Cao, Y.-X.; Zhou, X.; Wei, B. Delivery of folic acid-modified liposomal curcumin for targeted cervical carcinoma therapy. Drug Des. Devel. Ther. 2019, 13, 2205–2213. [Google Scholar] [CrossRef] [Green Version]
- Mahon, J.; Zhu, S. Interactions of poly(2-methacryloyloxyethyl phosphorylcholine) with various salts studied by size exclusion chromatography. Colloid. Polym. Sci. 2008, 286, 1443. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Xu, Q.; Xu, S.; Wen, J.; Yu, Z.; Yang, D. Folate-chitosan-gemcitabine core-shell nanoparticles targeted to pancreatic cancer. Chin. J. Cancer Res. 2013, 25, 527–535. [Google Scholar] [PubMed]
- Yin, J.-J.; Sharma, S.; Shumyak, S.P.; Wang, Z.-X.; Zhou, Z.-W.; Zhang, Y.; Guo, P.; Li, C.-Z.; Kanwar, J.R.; Yang, T.; et al. Synthesis and biological evaluation of novel folic acid receptor-targeted, β-cyclodextrin-based drug complexes for cancer treatment. PLoS ONE 2013, 8, e62289. [Google Scholar] [CrossRef] [Green Version]
- Pepić, I.; Lovrić, J.; Filipović-Grčić, J. How do polymeric micelles cross epithelial barriers. Eur. J. Pharm. Sci. 2013, 50, 42–55. [Google Scholar] [CrossRef] [PubMed]






| Micelles | FA conc. [g/g] | CMC c) | Size (PDI) d) | Zeta Potential d) | Size e) | LC/EE f) | |
|---|---|---|---|---|---|---|---|
| Theo.a) | Exp.b) | [mg/mL] | [nm] | [mV] | [nm] | [%] | |
| FPM1 | 0.034 | 0.012 | 2.5 × 10−3 | 162.7 ± 11.4 (0.24 ± 0.05) | −6.95 ± 2.85 | 159 ± 40 | - |
| CUR-FPM1 | 0.034 | 0.012 | - | 216.8 ± 4.8 (0.15 ± 0.09) | −5.32 ± 3.03 | 225 ± 32 | 6.3 ± 1.2/52.3 ± 9.9 |
| FPM2 | 0.025 | 0.022 | 6.0 × 10−4 | 192.7 ± 14.5 (0.12 ± 0.02) | −13.11 ± 11.58 | 172 ± 40 | - |
| CUR-FPM2 | 0.025 | 0.022 | - | 237.6 ± 31.7 (0.13 ± 0.05) | −10.6 ± 2.61 | 238 ± 44 | 5.7 ± 0.7/52.3 ± 9.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.H.; Chen, C.-Y. Folate-Targeted Curcumin-Encapsulated Micellar Nanosystem for Chemotherapy and Curcumin-Mediated Photodynamic Therapy. Polymers 2020, 12, 2280. https://doi.org/10.3390/polym12102280
Lin YH, Chen C-Y. Folate-Targeted Curcumin-Encapsulated Micellar Nanosystem for Chemotherapy and Curcumin-Mediated Photodynamic Therapy. Polymers. 2020; 12(10):2280. https://doi.org/10.3390/polym12102280
Chicago/Turabian StyleLin, Yun Hsuan, and Ching-Yi Chen. 2020. "Folate-Targeted Curcumin-Encapsulated Micellar Nanosystem for Chemotherapy and Curcumin-Mediated Photodynamic Therapy" Polymers 12, no. 10: 2280. https://doi.org/10.3390/polym12102280
APA StyleLin, Y. H., & Chen, C. -Y. (2020). Folate-Targeted Curcumin-Encapsulated Micellar Nanosystem for Chemotherapy and Curcumin-Mediated Photodynamic Therapy. Polymers, 12(10), 2280. https://doi.org/10.3390/polym12102280