Low Molecular Weight and Polymeric Modifiers as Toughening Agents in Poly(3-Hydroxybutyrate) Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biosynthesis of PHO
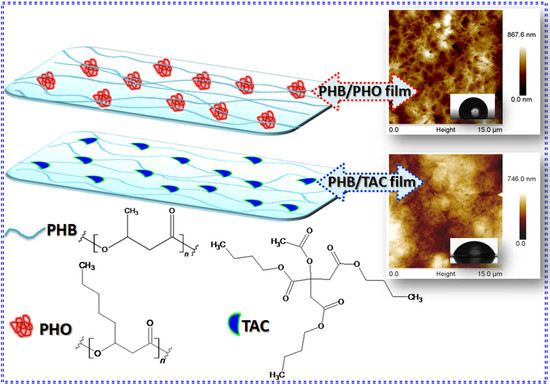
2.3. Preparation of PHB/PHO and PHB/TAC
2.4. Characterization
2.4.1. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis of PHO
2.4.2. Thermogravimetric Analysis (TGA)
2.4.3. Differential Scanning Calorimetry (DSC)
2.4.4. X-ray Diffraction (XRD)
2.4.5. Tensile Tests
2.4.6. Atomic Force Microscopy (AFM)
2.4.7. X-ray Photoelectron Spectroscopy (XPS)
2.4.8. Contact Angle Measurements (CA)
2.4.9. Biocompatibility Test
2.4.10. Evaluation of Pro-Inflammatory Effect
3. Results and Discussion
3.1. PHO Characterization
3.2. Thermal Stability of PHB Blends
3.3. DSC Analysis of Plasticized PHB
3.4. X-ray Diffraction (XRD)
3.5. Mechanical Characterization
3.6. Surface Morphology
3.7. Surface Properties by X-ray Photoelectron Spectroscopy
3.8. In Vitro Biocompatibility
3.9. Pro-Inflammatory Effect Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nielsen, T.D.; Hasselbalch, J.; Holmberg, K.; Stripple, J. Politics and the plastic crisis: A review throughout the plastic life cycle. WIREs Energy Environ. 2020, 9, 1–18. [Google Scholar]
- Torres-Giner, S.; Hilliou, L.; Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Madalena, D.; Cabedo, L.; Vicente, A.A.; Lagaron, J.M. Melt processability, characterization, and antibacterial activity of compression-molded green composite sheets made of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) reinforced with coconut fibers impregnated with oregano essential oil. Food Packag. Shelf Life 2018, 17, 39–49. [Google Scholar]
- Bonartsev, A.P.; Bonartseva, G.A.; Reshetov, I.V.; Kirpichniko, M.P.; Shaitan, K.V. Application of Polyhydroxyalkanoates in Medicine and the Biological Activity of Natural Poly(3-Hydroxybutyrate). Acta Nat. 2019, 11, 4–16. [Google Scholar]
- Mangeon, C.; Michely, L.; Rios de Anda, A.; Thevenieau, F.; Renard, E.; Langlois, V. Natural Terpenes Used as Plasticizers for Poly(3-hydroxybutyrate). ACS Sustain. Chem. Eng. 2018, 6, 16160–16168. [Google Scholar]
- Panaitescu, D.M.; Nicolae, C.A.; Gabor, A.R.; Trusca, R. Thermal and mechanical properties of poly(3-hydroxybutyrate) reinforced with cellulose fibers from wood waste. Ind. Crops Prod. 2020, 145. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Frone, A.N.; Chiulan, I. Nanostructured biocomposites from aliphatic polyesters and bacterial cellulose. Ind. Crops Prod. 2016, 93, 251–266. [Google Scholar]
- Audic, J.; Lemiègre, L.; Corre, Y. Thermal and mechanical properties of a polyhydroxyalkanoate plasticized with biobased epoxidized broccoli oil. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Erceg, M.; Kovacic, T.; Klaric, I. Thermal degradation of poly(3-hydroxybutyrate) plasticized with acetyl tributyl citrate. Polym. Degrad. Stab. 2005, 90, 313–318. [Google Scholar]
- Seoane, I.T.; Manfredi, L.B.; Cyras, V.P. Effect of two different plasticizers on the properties of poly(3-hydroxybutyrate) binary and ternary blends. J. Appl. Polym. Sci. 2017, 135, 46016. [Google Scholar]
- Wang, L.; Zhu, W.; Wang, X.; Chen, X.; Chen, G.Q.; Xu, K. Processability modifications of poly(3-hydroxybutyrate) by plasticizing, blending, and stabilizing. J. Appl. Polym. Sci. 2008, 107, 166–173. [Google Scholar]
- Requena, R.; Jiménez, A.; Vargas, M.; Chiralt, A. Effect of plasticizers on thermal and physical properties of compression-moulded poly[(3-hydroxybutyrate)-co-(3-hydroxyvalerate)] films. Polym. Test. 2016, 56, 45–53. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Fenollar, O.; Fombuena, V.; Lopez-Martinez, J.; Balart, R. Improvement of mechanical ductile properties of poly(3-hydroxybutyrate) by using vegetable oil derivatives. Macromol. Mater. Eng. 2017, 302. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Panariello, L.; Gigante, V.; Coltelli, M.-B.; Lazzeri, A. Sustainable Micro and Nano Additives for Controlling the Migration of a Biobased Plasticizer from PLA-Based Flexible Films. Polymers 2020, 12, 1366. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Development of flexible materials based on plasticized electrospun PLA–PHB blends: Structural, thermal, mechanical and disintegration properties. Eur. Polym. J. 2015, 73, 433–446. [Google Scholar] [CrossRef]
- Kurusu, R.S.; Siliki, C.A.; David, É.; Demarquette, N.R.; Gauthier, C.; Chenal, J.M. Incorporation of plasticizers in sugarcane-based poly(3-hydroxybutyrate)(PHB): Changes in microstructure and properties through ageing and annealing. Ind. Crops Prod. 2015, 72, 166–174. [Google Scholar] [CrossRef]
- Maiza, M.; Benaniba, M.T.; Quintard, G.; Massardier-Nageotte, V. Biobased additive plasticizing Polylactic acid (PLA). Polímeros 2015, 256, 581–590. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Nicolae, C.A.; Frone, A.N.; Chiulan, I.; Stanescu, P.O.; Draghici, C.; Iorga, M.; Mihailescu, M. Plasticized poly(3-hydroxybutyrate) with improved melt processing and balanced properties. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Biodegradable electrospunbionanocomposite fibers based on plasticized PLA–PHB blends reinforced with cellulose nanocrystals. Ind. Crops Prod. 2016, 93, 290–301. [Google Scholar] [CrossRef]
- Kang, H.; Li, Y.; Gong, M.; Guo, Y.; Guo, Z.; Fang, Q.; Li, X. An environmentally sustainable plasticizer toughened polylactide. RSC Adv. 2018, 8, 11643–11651. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, M.C.S.; Branciforti, M.C.; Pollet, E.; Agnelli, J.A.M.; Nascente, P.A.P.; Avérous, L. Elaboration and Characterization of Nano-Biocomposites Based on Plasticized Poly(Hydroxybutyrate-Co-Hydroxyvalerate) with Organo-Modified Montmorillonite. J. Polym. Environ. 2012, 20, 283–290. [Google Scholar] [CrossRef]
- Dias, A.M.A.; Marceneiro, S.; Braga, M.E.M.; Coelho, J.F.J.; Ferreira, A.G.M.; Simões, P.N.; Veiga, H.I.M.; Tomé, L.C.I.; Marrucho, M.; Esperança, J.M.S.S.; et al. Phosphonium-based ionic liquids as modifiers for biomedical grade poly(vinyl chloride). Acta Biomater. 2012, 8, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Nerkar, M.; Ramsay, J.A.; Ramsay, B.A.; Kontopoulou, M. Melt Compounded Blends of Short and Medium Chain-Length Poly-3-hydroxyalkanoates. J. Polym. Environ. 2014, 22, 236–243. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Lupescu, I.; Frone, A.N.; Chiulan, I.; Nicolae, C.A.; Tofan, V.; Stefaniu, A.; Somoghi, R.; Trusca, R. Medium Chain-Length Polyhydroxyalkanoate Copolymer Modified by Bacterial Cellulose for Medical Devices. Biomacromolecules 2017, 18, 3222–3232. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A.; Vincendon, M. Poly(3-hydroxybutyrate) and Poly(3-hydroxyoctanoate) Blends: Morphology and Mechanical Behavior. Macromolecules 2000, 33, 2998–3008. [Google Scholar] [CrossRef]
- Chiulan, I.; Panaitescu, D.M.; Frone, A.N.; Teodorescu, M.; Nicolae, C.A.; Casarica, A.; Tofan, V.; Salageanu, A. Biocompatible polyhydroxyalkanoates/bacterial cellulose composites: Preparation, characterization, and in vitro evaluation. J. Biomed. Mater. Res. A 2016, 104, 2576–2584. [Google Scholar] [CrossRef]
- Bragg, W.H.; Bragg, W.L. The reflexion of X-rays by crystals. Proc. R. Soc. London Ser. A 1913, 88, 428–438. [Google Scholar]
- Patterson, A.L. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Weinmann, S.; Bonten, C. Thermal and rheological properties of modified polyhydroxybutyrate (PHB). Polym. Eng. Sci. 2019, 59, 1057–1064. [Google Scholar] [CrossRef]
- Kim, K.J.; Doi, Y.; Abe, H. Effects of residual metal compounds and chain-end structure on thermal degradation of poly(3-hydroxybutyric acid). Polym. Degrad. Stab. 2006, 91, 769–777. [Google Scholar] [CrossRef]
- Kim, K.J.; Doi, Y.; Abe, H. Effect of metal compounds on thermal degradation behavior of aliphatic poly(hydroxyalkanoic acid)s. Polym. Degrad. Stab. 2008, 93, 776–785. [Google Scholar] [CrossRef]
- Anbukarasu, P.; Sauvageau, D.; Elias, A. Tuning the properties of polyhydroxybutyrate films using acetic acid via solvent casting. Sci. Rep. 2016, 5, 17884. [Google Scholar] [CrossRef] [Green Version]
- Malmir, S.; Montero, B.; Rico, M.; Barral, L.; Bouza, R. Morphology, thermal and barrier properties of biodegradable films of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) containing cellulose nanocrystals. Comp. Part A Appl. Sci. Manuf. 2017, 93, 41–48. [Google Scholar] [CrossRef]
- Siracusa, V.; Ingrao, C.; Karpova, S.G.; Olkhov, A.A.; Iordanskii, A.L. Gas transport and characterization of poly(3 hydroxybutyrate) films. Eur. Polym. J. 2017, 91, 149–161. [Google Scholar] [CrossRef]
- Prakalathan, K.; Mohanty, S.; Nayak, S.K. Reinforcing effect and isothermal crystallization kinetics of poly(3-hydroxybutyrate) nanocomposites blended with organically modified montmorillonite. Polym. Compos. 2014, 35, 999–1012. [Google Scholar] [CrossRef]
- Baltieri, R.C.; Innocentini Mei, L.H.; Bartoli, J. Study of the influence of plasticizers on the thermal and mechanical properties of poly(3-hydroxybutyrate) compounds. Macromol. Symp. 2003, 197, 33–44. [Google Scholar] [CrossRef]
- Iulianelli, G.C.V.; David, G.D.S.; dos Santos, T.N.; Sebastião, P.J.O.; Tavares, M.I.B. Influence of TiO2 nanoparticle on the thermal, morphological and molecular characteristics of PHB matrix. Polym. Test. 2018, 65, 156–162. [Google Scholar] [CrossRef]
- Chen, J.; Wu, D.; Tam, K.C.; Pan, K.; Zheng, Z. Effect of surface modification of cellulose nanocrystal on nonisothermal crystallization of poly(β-hydroxybutyrate) composites. Carbohydr. Polym. 2017, 157, 1821–1829. [Google Scholar] [CrossRef]
- Hong, S.G.; Gau, T.K.; Huang, S.C. Enhancement of thecrystallization and thermal stability of polyhydroxybutyratebypolymericadditives. J. Therm. Anal. Calorim. 2011, 103, 967–975. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Ferri, J.M.; Montanes, N.; Lopez-Martinez, J.; Balart, R. Plasticization effects of epoxidized vegetable oils on mechanical properties of poly(3-hydroxybutyrate). Polym. Int. 2016, 65, 1157–1164. [Google Scholar] [CrossRef]
- Lin, K.W.; Lan, C.H.; Sun, Y.M. Poly[(R)3-hydroxybutyrate] (PHB)/poly(l-lactic acid) (PLLA) blends with poly(PHB/PLLA urethane) as a compatibilizer. Polym. Degrad. Stab. 2016, 134, 30–40. [Google Scholar] [CrossRef]
- Lee, C.W.; Song, B.K.; Jegal, J.; Kimura, Y. Cell adhesion and surface chemistry of biodegradable aliphatic polyesters: Discovery of particularly low cell adhesion behavior on poly(3-[RS]-hydroxybutyrate). Macromol. Res. 2013, 21, 1305–1313. [Google Scholar] [CrossRef]
- Sofińska, K.; Barbasz, J.; Witko, T.; Dryzek, J.; Haraźna, K.; Witko, M.; Kryściak-Czerwenka, J.; Guzik, M. Structural, topographical, and mechanical characteristics of purified polyhydroxyoctanoate polymer. J. Appl. Polym. Sci. 2019, 136, 47192. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Frone, A.N.; Chiulan, I.; Casarica, A.; Nicolae, C.A.; Ghiurea, M.; Trusca, R.; Damian, C.M. Structural and morphological characterization of bacterial cellulose nano-reinforcements prepared by mechanical route. Mater. Des. 2016, 110, 790–801. [Google Scholar] [CrossRef]
- Wang, C.; Sauvageau, D.; Elias, A. Immobilization of Active Bacteriophages on Polyhydroxyalkanoate Surfaces. ACS Appl. Mater. Interfaces 2016, 8, 1128–1138. [Google Scholar] [CrossRef]
- da Silva, M.G.; Vargas, H.; Poley, L.H.; Rodriguez, R.S.; Baptista, G.B. Structural impact of hydroxyvalerate in polyhydroxyalkanoates (PHAscl) dense film monitored by XPS and photothermal methods. J. Braz. Chem. Soc. 2005, 164, 790–795. [Google Scholar] [CrossRef]
- Lee, C.W.; Horiike, M.; Masutani, K.; Kimura, Y. Characteristic cell adhesion behaviors on various derivatives of poly(3-hydroxybutyrate) (PHB) and a block copolymer of poly(3-[RS]-hydroxybutyrate) and poly(oxyethylene). Polym. Degrad. Stab. 2015, 111, 194–202. [Google Scholar] [CrossRef]
- Qu, X.H.; Wu, Q.; Liang, J.; Zou, B.; Chen, G.Q. Effect of 3-hydroxyhexanoate content in poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) on in vitro growth and differentiation of smooth muscle cells. Biomaterials 2006, 27, 2944–2950. [Google Scholar] [CrossRef]
- Tamada, Y.; Ikada, Y. Fibroblast growth on polymer surfaces and biosynthesis of collagen. J. Biomed. Mater. Res. 1994, 28, 783–789. [Google Scholar] [CrossRef]
- Boyan, B.D.; Hummert, T.W.; Dean, D.D.; Schwartz, Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 1996, 17, 137–146. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Chernozem, R.V.; Syromotina, D.S.; Oehr, C.; Baumbach, T.; Krause, B.; Boyandin, A.N.; Dvoinina, L.M.; Volova, T.G.; Surmeneva, M.A. Low-temperature argon and ammonia plasma treatment of poly-3-hydroxybutyrate films: Surface topography and chemistry changes affect fibroblast cells in vitro. Eur. Polym. J. 2019, 112, 137–145. [Google Scholar] [CrossRef] [Green Version]











| Sample | T5% (°C) | WL220 (%) | Td1 (°C) | Td2 (°C) | R500 * (%) |
|---|---|---|---|---|---|
| PHB | 261.3 | 0.3 | - | 281.4 | 0.7 |
| PHB/5PHO | 252.2 | 0.4 | - | 275.0 | 0.7 |
| PHB/10PHO | 245.6 | 0.5 | - | 269.3 | 0.5 |
| PHB/15PHO | 246.6 | 1.1 | - | 271.0 | 0.7 |
| PHB/20PHO | 227.5 | 2.2 | - | 255.0 | 1.1 |
| PHO | 262.3 | 0.2 | - | 287.8 | 2.8 |
| PHB/5TAC | 213.1 | 5.0 | 202.5 | 275.5 | 0.4 |
| PHB/10TAC | 180.8 | 7.0 | 191.1 | 279.5 | 0.1 |
| PHB/15TAC | 174.6 | 17.0 | 195.9 | 277.4 | 0.3 |
| PHB/20TAC | 176.1 | 17.0 | 198.5 | 278.2 | 0.3 |
| Sample | Heating | Cooling | ||||
|---|---|---|---|---|---|---|
| Tm1/Tm2 (°C) | ΔH1/ΔH2 (J/g) | Xc (%) | Tg (°C) | Tc (°C) | ΔHc (J/g) | |
| PHB | 163.3/173.6 | 17.8/59.3 | 53 | 7.1 | 88.9 | 59.0 |
| PHB/5PHO | 164.2/173.7 | 21.1/52.0 | 53 | 4.7 | 82.4 | 52.4 |
| PHB/10PHO | 163.03/173.3 | 16.7/52.4 | 53 | 6.6 | 84.4 | 50.6 |
| PHB/20PHO | 163.9/173.0 | 18.9/41.9 | 52 | 4.0 | 75.6 | 40.9 |
| PHB/5TAC | 158.3/172.1 | 14.5/58.4 | 53 | 4.5 | 83.6 | 51.6 |
| PHB/10TAC | 153.8/169.9 | 11.9/58.7 | 54 | −1.5 | 76.5 | 49.5 |
| PHB/20TAC | 150.0/169.2 | 7.6/61.9 | 60 | −1.5 | 77.0 | 51.2 |
| Sample | d020 (nm) | d110 (nm) | d021 (nm) | d101 (nm) | d111 (nm) | d121 (nm) | d040 (nm) | d002 (nm) | D020 (nm) | D110 (nm) | D040 (nm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PHB | 0.6565 | 0.5259 | 0.4426 | 0.4140 | 0.3948 | 0.3497 | 0.3282 | 0.2928 | 14.8 | 7.5 | 8.8 |
| PHB/20PHO | 0.6580 | 0.5253 | 0.4428 | 0.4123 | 0.3927 | 0.3498 | 0.3279 | 0.2958 | 17.4 | 13.3 | 14.5 |
| PHB/20TAC | 0.6565 | 0.5240 | 0.4426 | 0.4118 | 0.3922 | 0.3597 | 0.3279 | 0.2923 | 15.9 | 13.3 | 20.4 |
| Sample | Atomic Percentage (%) | C1s Peak Fit Atomic Percentage (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| C1s | O1s | C/O Experimental | C/O Theoretical | Difference | C1 [C-C] | C2 [C-O] | C3 [C=O] | |
| PHB | 69.99 | 30.01 | 2.33 | 2.00 | +0.33 | 51.89 | 25.16 | 22.95 |
| PHB/5PHO | 70.19 | 29.81 | 2.35 | 2.10 | +0.25 | 52.81 | 25.67 | 21.52 |
| PHB/10PHO | 70.90 | 29.10 | 2.44 | 2.20 | +0.20 | 55.46 | 23.89 | 20.64 |
| PHB/20PHO | 70.82 | 29.18 | 2.43 | 2.40 | +0.03 | 57.96 | 22.10 | 19.94 |
| PHB/5TAC | 70.49 | 29.51 | 2.39 | 2.03 | +0.36 | 50.91 | 26.05 | 23.03 |
| PHB/10TAC | 69.89 | 30.11 | 2.32 | 2.05 | +0.27 | 50.22 | 26.63 | 23.15 |
| PHB/20TAC | 70.39 | 29.61 | 2.38 | 2.10 | +0.28 | 50.77 | 26.37 | 22.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frone, A.N.; Nicolae, C.A.; Eremia, M.C.; Tofan, V.; Ghiurea, M.; Chiulan, I.; Radu, E.; Damian, C.M.; Panaitescu, D.M. Low Molecular Weight and Polymeric Modifiers as Toughening Agents in Poly(3-Hydroxybutyrate) Films. Polymers 2020, 12, 2446. https://doi.org/10.3390/polym12112446
Frone AN, Nicolae CA, Eremia MC, Tofan V, Ghiurea M, Chiulan I, Radu E, Damian CM, Panaitescu DM. Low Molecular Weight and Polymeric Modifiers as Toughening Agents in Poly(3-Hydroxybutyrate) Films. Polymers. 2020; 12(11):2446. https://doi.org/10.3390/polym12112446
Chicago/Turabian StyleFrone, Adriana Nicoleta, Cristian Andi Nicolae, Mihaela Carmen Eremia, Vlad Tofan, Marius Ghiurea, Ioana Chiulan, Elena Radu, Celina Maria Damian, and Denis Mihaela Panaitescu. 2020. "Low Molecular Weight and Polymeric Modifiers as Toughening Agents in Poly(3-Hydroxybutyrate) Films" Polymers 12, no. 11: 2446. https://doi.org/10.3390/polym12112446
APA StyleFrone, A. N., Nicolae, C. A., Eremia, M. C., Tofan, V., Ghiurea, M., Chiulan, I., Radu, E., Damian, C. M., & Panaitescu, D. M. (2020). Low Molecular Weight and Polymeric Modifiers as Toughening Agents in Poly(3-Hydroxybutyrate) Films. Polymers, 12(11), 2446. https://doi.org/10.3390/polym12112446