Polymeric Drug Delivery Systems Bearing Cholesterol Moieties: A Review
Abstract
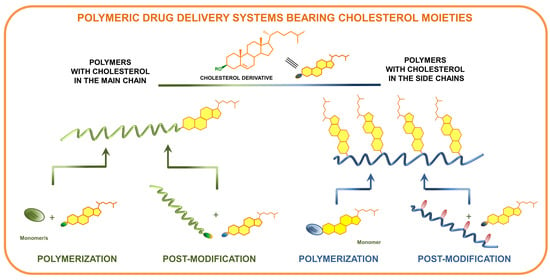
:1. Introduction
2. Methods of Synthesis of Polymers Containing Cholesterol
2.1. Polymers Containing Cholesterol in the Main Chain
2.1.1. Cholesterol Introduced to the Main Chain during Polymerization
2.1.2. Cholesterol Introduced to the Main Chain by Post-Modification
2.2. Polymers Containing Cholesterol as Side Chains
2.2.1. Polymers Containing Cholesterol Moieties as Side Chains Obtained by Polymerization of Cholesterol-Based Monomers
2.2.2. Polymers Bearing Cholesterol Moieties as Side Chains Obtained by Post-Modification
3. Form of Carriers
4. Drug Encapsulation and Release
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [Green Version]
- Jelonek, K.; Kasperczyk, J. Polyesters and polyester carbonates for controlled drug delivery. Part II. Implantable systems. Polimery 2013, 58, 858–863. [Google Scholar] [CrossRef]
- Jones, D. Pharmaceutical Applications of Polymers for Drug Delivery; Rapra Review Reports; Rapra Technology: Shrewsbury, UK, 2004; ISBN 978-1-85957-479-9. [Google Scholar]
- Nakielski, P. Drug Release Systems Based on Nanofibers; IPPT Reports on Fundamental Technological Research; IPPT PAN: Warsaw, Poland, 2015; ISBN 978-83-89687-93-7. [Google Scholar]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef]
- El-Sawy, H.S.; Al-Abd, A.M.; Ahmed, T.A.; El-Say, K.M.; Torchilin, V.P. Stimuli-Responsive Nano-Architecture Drug-Delivery Systems to Solid Tumor Micromilieu: Past, Present, and Future Perspectives. ACS Nano 2018, 12, 10636–10664. [Google Scholar] [CrossRef]
- Li, B.L.; Li, R.; Zou, H.L.; Ariga, K.; Li, N.B.; Leong, D.T. Engineered functionalized 2D nanoarchitectures for stimuli-responsive drug delivery. Mater. Horiz. 2020, 7, 455–469. [Google Scholar] [CrossRef]
- An, H.; Li, M.; Gao, J.; Zhang, Z.; Ma, S.; Chen, Y. Incorporation of biomolecules in Metal-Organic Frameworks for advanced applications. Coord. Chem. Rev. 2019, 384, 90–106. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Mukherjee, S. Fluorescent Metal Nano-Clusters as Next Generation Fluorescent Probes for Cell Imaging and Drug Delivery. Bull. Chem. Soc. Jpn. 2018, 91, 447–454. [Google Scholar] [CrossRef]
- Cheng, L.-C.; Jiang, Y.; Xie, Y.; Qiu, L.-L.; Yang, Q.; Lu, H.-Y. Novel amphiphilic folic acid-cholesterol-chitosan micelles for paclitaxel delivery. Oncotarget 2017, 8, 3315–3326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Liu, Y.; Yang, W.; Li, X.; Liu, L.; Zhou, Z.; Wang, Y.; Li, R.; Zhang, Q. Preparation and characterization of self-assembled nanoparticles of 6-O-cholesterol-modified chitosan for drug delivery. Carbohydr. Polym. 2011, 84, 1244–1251. [Google Scholar] [CrossRef]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Nakai, T.; Hirakura, T.; Sakurai, Y.; Shimoboji, T.; Ishigai, M.; Akiyoshi, K. Injectable Hydrogel for Sustained Protein Release by Salt-Induced Association of Hyaluronic Acid Nanogel. Macromol. Biosci. 2012, 12, 475–483. [Google Scholar] [CrossRef]
- Huerta-Ángeles, G.; Brandejsová, M.; Novotný, J.; Kopecká, K.; Šógorková, J.; Šmejkalová, D.; Velebný, V. Grafting of steroids to hyaluronan towards the design of delivery systems for antioxidants: The role of hydrophobic core. Carbohydr. Polym. 2018, 193, 383–392. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, D.; Li, Y.; Yang, X.; Pan, W. In vitro–in vivo evaluation of hyaluronic acid-based amphiphilic copolymers for tumour targeted delivery: The role of hydrophobic groups. RSC Adv. 2017, 7, 23942–23953. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Li, Y.; Yang, X.; Pan, W.; Pan, H. The reversion of anti-cancer drug antagonism of tamoxifen and docetaxel by the hyaluronic acid-decorated polymeric nanoparticles. Pharmacol. Res. 2017, 126, 84–96. [Google Scholar] [CrossRef]
- Fukunaga, K.; Tsutsumi, H.; Mihara, H. Self-Assembling Peptides as Building Blocks of Functional Materials for Biomedical Applications. Bull. Chem. Soc. Jpn. 2019, 92, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Jäger, E.; Jäger, A.; Chytil, P.; Etrych, T.; Říhová, B.; Giacomelli, F.C.; Štěpánek, P.; Ulbrich, K. Combination chemotherapy using core-shell nanoparticles through the self-assembly of HPMA-based copolymers and degradable polyester. J. Control. Release 2013, 165, 153–161. [Google Scholar] [CrossRef]
- Chytil, P.; Šírová, M.; Kudláčová, J.; Říhová, B.; Ulbrich, K.; Etrych, T. Bloodstream Stability Predetermines the Antitumor Efficacy of Micellar Polymer–Doxorubicin Drug Conjugates with pH-Triggered Drug Release. Mol. Pharm. 2018, 15, 3654–3663. [Google Scholar] [CrossRef]
- Filippov, S.K.; Franklin, J.M.; Konarev, P.V.; Chytil, P.; Etrych, T.; Bogomolova, A.; Dyakonova, M.; Papadakis, C.M.; Radulescu, A.; Ulbrich, K.; et al. Hydrolytically Degradable Polymer Micelles for Drug Delivery: A SAXS/SANS Kinetic Study. Biomacromolecules 2013, 14, 4061–4070. [Google Scholar] [CrossRef]
- Chytil, P.; Etrych, T.; Kostka, L.; Ulbrich, K. Hydrolytically Degradable Polymer Micelles for Anticancer Drug Delivery to Solid Tumors. Macromol. Chem. Phys. 2012, 213, 858–867. [Google Scholar] [CrossRef]
- Zhang, X.; Niebuur, B.-J.; Chytil, P.; Etrych, T.; Filippov, S.K.; Kikhney, A.; Wieland, D.C.F.; Svergun, D.I.; Papadakis, C.M. Macromolecular p HPMA-Based Nanoparticles with Cholesterol for Solid Tumor Targeting: Behavior in HSA Protein Environment. Biomacromolecules 2018, 19, 470–480. [Google Scholar] [CrossRef]
- Van Elk, M.; Deckers, R.; Oerlemans, C.; Shi, Y.; Storm, G.; Vermonden, T.; Hennink, W.E. Triggered Release of Doxorubicin from Temperature-Sensitive Poly(N-(2-hydroxypropyl)-methacrylamide mono/dilactate) Grafted Liposomes. Biomacromolecules 2014, 15, 1002–1009. [Google Scholar] [CrossRef]
- Chiang, Y.-T.; Lo, C.-L. pH-Responsive polymer-liposomes for intracellular drug delivery and tumor extracellular matrix switched-on targeted cancer therapy. Biomaterials 2014, 35, 5414–5424. [Google Scholar] [CrossRef]
- Chiang, Y.-T.; Cheng, Y.-T.; Lu, C.-Y.; Yen, Y.-W.; Yu, L.-Y.; Yu, K.-S.; Lyu, S.-Y.; Yang, C.-Y.; Lo, C.-L. Polymer–Liposome Complexes with a Functional Hydrogen-Bond Cross-Linker for Preventing Protein Adsorption and Improving Tumor Accumulation. Chem. Mater. 2013, 25, 4364–4372. [Google Scholar] [CrossRef]
- Lou, B.; Connor, K.; Sweeney, K.; Miller, I.S.; O’Farrell, A.; Ruiz-Hernandez, E.; Murray, D.M.; Duffy, G.P.; Wolfe, A.; Mastrobattista, E.; et al. RGD-decorated cholesterol stabilized polyplexes for targeted siRNA delivery to glioblastoma cells. Drug Deliv. Transl. Res. 2019, 9, 679–693. [Google Scholar] [CrossRef] [Green Version]
- Kumari, P.; Muddineti, O.S.; Rompicharla, S.V.K.; Ghanta, P.; B B N, A.K.; Ghosh, B.; Biswas, S. Cholesterol-conjugated poly(d,l-lactide)-based micelles as a nanocarrier system for effective delivery of curcumin in cancer therapy. Drug Deliv. 2017, 24, 209–223. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Lin, D.; Wu, F.; Guo, L.; He, G.; Ouyang, L.; Song, X.; Huang, W.; Li, X. Discovery and in Vivo Evaluation of Novel RGD-Modified Lipid-Polymer Hybrid Nanoparticles for Targeted Drug Delivery. Int. J. Mol. Sci. 2014, 15, 17565–17576. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.; Marino, N.; Curran, C.; McHale, A.P.; Callan, J.F.; Callan, B. Cholesteryl to improve the cellular uptake of polymersomes within HeLa cells. Int. J. Pharm. 2016, 511, 570–578. [Google Scholar] [CrossRef]
- Venkataraman, S.; Lee, A.L.; Maune, H.T.; Hedrick, J.L.; Prabhu, V.M.; Yang, Y.Y. Formation of Disk- and Stacked-Disk-like Self-Assembled Morphologies from Cholesterol-Functionalized Amphiphilic Polycarbonate Diblock Copolymers. Macromolecules 2013, 46, 4839–4846. [Google Scholar] [CrossRef]
- Lee, A.L.Z.; Venkataraman, S.; Sirat, S.B.M.; Gao, S.; Hedrick, J.L.; Yang, Y.Y. The use of cholesterol-containing biodegradable block copolymers to exploit hydrophobic interactions for the delivery of anticancer drugs. Biomaterials 2012, 33, 1921–1928. [Google Scholar] [CrossRef]
- Gonzalez-Fajardo, L.; Mahajan, L.H.; Ndaya, D.; Hargrove, D.; Manautou, J.E.; Liang, B.T.; Chen, M.-H.; Kasi, R.M.; Lu, X. Reduced in vivo toxicity of doxorubicin by encapsulation in cholesterol-containing self-assembled nanoparticles. Pharmacol. Res. 2016, 107, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.-H.; Nguyen, C.T.; Gonzalez-Fajardo, L.; Hargrove, D.; Song, D.; Deshmukh, P.; Mahajan, L.; Ndaya, D.; Lai, L.; Kasi, R.M.; et al. Long Circulating Self-Assembled Nanoparticles from Cholesterol-Containing Brush-Like Block Copolymers for Improved Drug Delivery to Tumors. Biomacromolecules 2014, 15, 4363–4375. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Cui, D.; Bignon, J.; Di Cicco, A.; Wdzieczak-Bakala, J.; Liu, J.; Li, M.-H. Reduction-Responsive Cholesterol-Based Block Copolymer Vesicles for Drug Delivery. Biomacromolecules 2014, 15, 2206–2217. [Google Scholar] [CrossRef]
- Laskar, P.; Samanta, S.; Ghosh, S.K.; Dey, J. In vitro evaluation of pH-sensitive cholesterol-containing stable polymeric micelles for delivery of camptothecin. J. Colloid Interface Sci. 2014, 430, 305–314. [Google Scholar] [CrossRef]
- Monajati, M.; Tavakoli, S.; Abolmaali, S.S.; Tamaddon, A. Effect of PEGylation on assembly morphology and cellular uptake of poly ethyleneimine-cholesterol conjugates for delivery of sorafenib tosylate in hepatocellular carcinoma. BioImpacts 2018, 8, 241–252. [Google Scholar] [CrossRef]
- Yang, B.; Lv, Y.; Zhu, J.; Han, Y.; Jia, H.; Chen, W.; Feng, J.; Zhang, X.; Zhuo, R. A pH-responsive drug nanovehicle constructed by reversible attachment of cholesterol to PEGylated poly(l-lysine) via catechol–boronic acid ester formation. Acta Biomater. 2014, 10, 3686–3695. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.J.; Wang, Y.; Chen, B.Z.; Guo, X.D.; Zhang, C.Y. Dual redox/pH-responsive hybrid polymer-lipid composites: Synthesis, preparation, characterization and application in drug delivery with enhanced therapeutic efficacy. Chem. Eng. J. 2018, 341, 450–461. [Google Scholar] [CrossRef]
- Cheng, W.; Kumar, J.N.; Zhang, Y.; Liu, Y. pH- and Redox-Responsive Poly(ethylene glycol) and Cholesterol-Conjugated Poly(amido amine)s Based Micelles for Controlled Drug Delivery: PH- and Redox-Responsive Poly(amido amine)s Micelles for Controlled Drug Delivery. Macromol. Biosci. 2014, 14, 347–358. [Google Scholar] [CrossRef]
- Huang, X.; Liao, W.; Zhang, G.; Kang, S.; Zhang, C.Y. pH-sensitive micelles self-assembled from polymer brush (PAE-g-cholesterol)-b-PEG-b-(PAE-g-cholesterol) for anticancer drug delivery and controlled release. Int. J. Nanomedicine 2017, 12, 2215–2226. [Google Scholar] [CrossRef] [Green Version]
- Ghanbarzadeh, S.; Arami, S.; Pourmoazzen, Z.; Khorrami, A. Improvement of the antiproliferative effect of Rapamycin on tumor cell lines by poly(monomethylitaconate)-based pH-sensitive, plasma stable liposomes. Colloids Surf. B Biointerfaces 2014, 115, 323–330. [Google Scholar] [CrossRef]
- Ghanbarzadeh, S.; Arami, S.; Pourmoazzen, Z.; Ghasemian-Yadegari, J.; Khorrami, A. Plasma stable, pH-sensitive fusogenic polymer-modified liposomes: A promising carrier for mitoxantrone. J. Biomater. Appl. 2014, 29, 81–92. [Google Scholar] [CrossRef]
- Pourmoazzen, Z.; Bagheri, M.; Entezami, A.A.; Koshki, K.N. pH-responsive micelles composed of poly(ethylene glycol) and cholesterol-modified poly(monomethyl itaconate) as a nanocarrier for controlled and targeted release of piroxicam. J. Polym. Res. 2013, 20, 295. [Google Scholar] [CrossRef]
- Fritz, T.; Voigt, M.; Worm, M.; Negwer, I.; Müller, S.S.; Kettenbach, K.; Ross, T.L.; Roesch, F.; Koynov, K.; Frey, H.; et al. Orthogonal Click Conjugation to the Liposomal Surface Reveals the Stability of the Lipid Anchorage as Crucial for Targeting. Chem. Eur. J. 2016, 22, 11578–11582. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, C.Y.; Wu, J.; Zhou, H.; Bai, R.; Shen, Z.; Deng, F.; Liu, Y.; Liu, J. PEG-Detachable Polymeric Micelles Self-Assembled from Amphiphilic Copolymers for Tumor-Acidity-Triggered Drug Delivery and Controlled Release. ACS Appl. Mater. Interfaces 2019, 11, 5701–5713. [Google Scholar] [CrossRef]
- Sharma, S.; Mazumdar, S.; Italiya, K.S.; Date, T.; Mahato, R.I.; Mittal, A.; Chitkara, D. Cholesterol and Morpholine Grafted Cationic Amphiphilic Copolymers for miRNA-34a Delivery. Mol. Pharm. 2018, 15, 2391–2402. [Google Scholar] [CrossRef]
- Yuan, H.; Zhong, W.; Wang, R.; Zhou, P.; Nie, Y.; Hu, W.; Tao, X.; Yang, P. Preparation of Cholesteryl-Modified Aminated Pullulan Nanoparticles to Evaluate Nanoparticle of Hydrophobic Degree on Drug Release and Cytotoxicity. J. Nanomater. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.Y.; Wu, W.S.; Yao, N.; Zhao, B.; Zhang, L.J. pH-sensitive amphiphilic copolymer brush Chol-g-P(HEMA-co-DEAEMA)-b-PPEGMA: Synthesis and self-assembled micelles for controlled anti-cancer drug release. RSC Adv. 2014, 4, 40232–40240. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.Y.; Xiong, D.; Sun, Y.; Zhao, B.; Lin, W.J. Self-assembled micelles based on pH-sensitive PAE-g-MPEG-cholesterol block copolymer for anticancer drug delivery. Int. J. Nanomed. 2014, 4923. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, M.; Shateri, S.; Niknejad, H.; Entezami, A.A. Thermosensitive biotinylated hydroxypropyl cellulose-based polymer micelles as a nano-carrier for cancer-targeted drug delivery. J. Polym. Res. 2014, 21, 567. [Google Scholar] [CrossRef]
- Yang, C.; Liu, S.Q.; Venkataraman, S.; Gao, S.J.; Ke, X.; Chia, X.T.; Hedrick, J.L.; Yang, Y.Y. Structure-directing star-shaped block copolymers: Supramolecular vesicles for the delivery of anticancer drugs. J. Control. Release 2015, 208, 93–105. [Google Scholar] [CrossRef]
- Chen, H.-H.; Lu, I.-L.; Liu, T.-I.; Tsai, Y.-C.; Chiang, W.-H.; Lin, S.-C.; Chiu, H.-C. Indocyanine green/doxorubicin-encapsulated functionalized nanoparticles for effective combination therapy against human MDR breast cancer. Colloids Surf. B Biointerfaces 2019, 177, 294–305. [Google Scholar] [CrossRef]
- Gao, M.; Yang, Y.; Bergfel, A.; Huang, L.; Zheng, L.; Bowden, T.M. Self-assembly of cholesterol end-capped polymer micelles for controlled drug delivery. J. Nanobiotechnol. 2020, 18. [Google Scholar] [CrossRef] [Green Version]
- Andrén, O.C.J.; Zhang, Y.; Lundberg, P.; Hawker, C.J.; Nyström, A.M.; Malkoch, M. Therapeutic Nanocarriers via Cholesterol Directed Self-Assembly of Well-Defined Linear-Dendritic Polymeric Amphiphiles. Chem. Mater. 2017, 29, 3891–3898. [Google Scholar] [CrossRef]
- Yu, Y.; He, Y.; Xu, B.; He, Z.; Zhang, Y.; Chen, Y.; Yang, Y.; Xie, Y.; Zheng, Y.; He, G.; et al. Self-Assembled Methoxy Poly(Ethylene Glycol)-Cholesterol Micelles for Hydrophobic Drug Delivery. J. Pharm. Sci. 2013, 102, 1054–1062. [Google Scholar] [CrossRef]
- Zeng, S.; Wu, F.; Li, B.; Song, X.; Zheng, Y.; He, G.; Peng, C.; Huang, W. Synthesis, Characterization, and Evaluation of a Novel Amphiphilic Polymer RGD-PEG-Chol for Target Drug Delivery System. Sci. World J. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Villamil, J.C.; Parra-Giraldo, C.M.; Pérez, L.D. Enhancing the performance of PEG-b-PCL copolymers as precursors of micellar vehicles for amphotericin B through its conjugation with cholesterol. Colloids Surf. Physicochem. Eng. Asp. 2019, 572, 79–87. [Google Scholar] [CrossRef]
- Chen, Z.-P.; Xiao, L.; Liu, D.; Feng, M.-S.; Xiao, Y.-Y.; Chen, J.; Li, W.; Li, W.; Cai, B. Synthesis of a novel polymer cholesterol-poly(ethylene glycol) 2000-glycyrrhetinic acid (chol-PEG-GA) and its application in brucine liposome. J. Appl. Polym. Sci. 2011, 124, 4554–4563. [Google Scholar] [CrossRef]
- Li, J.; He, Z.; Yu, S.; Li, S.; Ma, Q.; Yu, Y.; Zhang, J.; Li, R.; Zheng, Y.; He, G.; et al. Micelles Based on Methoxy Poly(Ethylene Glycol)Cholesterol Conjugate for Controlled and Targeted Drug Delivery of a Poorly Water Soluble Drug. J. Biomed. Nanotechnol. 2012, 8, 809–817. [Google Scholar] [CrossRef]
- Kim, J.H.; Li, Y.; Kim, M.S.; Kang, S.W.; Jeong, J.H.; Lee, D.S. Synthesis and evaluation of biotin-conjugated pH-responsive polymeric micelles as drug carriers. Int. J. Pharm. 2012, 427, 435–442. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Ding, J.; Lu, S.; Wang, X.; Wang, C.; Chen, X. Cholesterol-Enhanced Polylactide-Based Stereocomplex Micelle for Effective Delivery of Doxorubicin. Materials 2015, 8, 216–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Xu, W.; Li, S.; Qiu, H.; Li, Z.; Wang, C.; Wang, X.; Ding, J. Polylactide-Cholesterol Stereocomplex Micelle Encapsulating Chemotherapeutic Agent for Improved Antitumor Efficacy and Safety. J. Biomed. Nanotechnol. 2018, 14, 2102–2113. [Google Scholar] [CrossRef]
- Cai, L.; Qiu, N.; Li, X.; Luo, K.; Chen, X.; Yang, L.; He, G.; Wei, Y.; Chen, L. A novel truncated basic fibroblast growth factor fragment-conjugated poly (ethylene glycol)-cholesterol amphiphilic polymeric drug delivery system for targeting to the FGFR-overexpressing tumor cells. Int. J. Pharm. 2011, 408, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, F.; Li, J.; Jiang, X.; Cai, L.; Li, X. DUP1 peptide modified micelle efficiently targeted delivery paclitaxel and enhance mitochondrial apoptosis on PSMA-negative prostate cancer cells. SpringerPlus 2016, 5, 362. [Google Scholar] [CrossRef] [Green Version]
- Vabbilisetty, P.; Sun, X.-L. Liposome surface functionalization based on different anchoring lipids via Staudinger ligation. Org. Biomol. Chem. 2014, 12, 1237. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.-R.; Zhu, Y.-X.; Xu, K.-F.; Liu, X.; Wu, F.-G. Plasma membrane-anchorable photosensitizing nanomicelles for lipid raft-responsive and light-controllable intracellular drug delivery. J. Control. Release 2018, 286, 103–113. [Google Scholar] [CrossRef]
- Oba, M.; Miyata, K.; Osada, K.; Christie, R.J.; Sanjoh, M.; Li, W.; Fukushima, S.; Ishii, T.; Kano, M.R.; Nishiyama, N.; et al. Polyplex micelles prepared from ω-cholesteryl PEG-polycation block copolymers for systemic gene delivery. Biomaterials 2011, 32, 652–663. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Vanaparthi, A.; Rompicharla, S.V.K.; Kumari, P.; Ghosh, B.; Biswas, S. Cholesterol and vitamin E-conjugated PEGylated polymeric micelles for efficient delivery and enhanced anticancer activity of curcumin: Evaluation in 2D monolayers and 3D spheroids. Artif. Cells Nanomed. Biotechnol. 2018, 46, 773–786. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Chen, Q.; Wu, W.S.; Guo, X.D.; Cai, C.Z.; Zhang, L.J. Synthesis and evaluation of cholesterol-grafted PEGylated peptides with pH-triggered property as novel drug carriers for cancer chemotherapy. Colloids Surf. B Biointerfaces 2016, 142, 55–64. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, C.; Li, M.; Ding, J.; Yang, C.; Zhuang, X.; Chen, X. Co-delivery of doxorubicin and paclitaxel with linear-dendritic block copolymer for enhanced anti-cancer efficacy. Sci. China Chem. 2014, 57, 624–632. [Google Scholar] [CrossRef]
- Barve, A.; Jain, A.; Liu, H.; Zhao, Z.; Cheng, K. Enzyme-responsive polymeric micelles of cabazitaxel for prostate cancer targeted therapy. Acta Biomater. 2020, S1742706120303494. [Google Scholar] [CrossRef]
- Chen, D. pH and temperature dual-sensitive liposome gel based on novel cleavable mPEG-Hz-CHEMS polymeric vaginal delivery system. Int. J. Nanomed. 2012, 2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanamala, M.; Palmer, B.D.; Ghandehari, H.; Wilson, W.R.; Wu, Z. PEG-Benzaldehyde-Hydrazone-Lipid Based PEG-Sheddable pH-Sensitive Liposomes: Abilities for Endosomal Escape and Long Circulation. Pharm. Res. 2018, 35, 154. [Google Scholar] [CrossRef]
- Kanamala, M.; Palmer, B.D.; Wilson, W.R.; Wu, Z. Characterization of a smart pH-cleavable PEG polymer towards the development of dual pH-sensitive liposomes. Int. J. Pharm. 2018, 548, 288–296. [Google Scholar] [CrossRef]
- Cho, S.-H.; Hong, J.H.; Noh, Y.-W.; Lee, E.; Lee, C.-S.; Lim, Y.T. Raspberry-like poly(gamma;-glutamic acid) hydrogel particles for pH-dependent cell membrane passage and controlled cytosolic delivery of antitumor drugs. Int. J. Nanomedicine 2016, 11, 5621–5632. [Google Scholar] [CrossRef] [Green Version]
- Lyu, X.; Zhang, Q.; Liang, D.; Huang, Y. Interaction between human serum albumin and cholesterol-grafted polyglutamate as the potential carriers of protein drugs. Acta Pharm. Sin. B 2019, 9, 186–193. [Google Scholar] [CrossRef]
- Terao, K.; Miyake, J.; Watanabe, J.; Ikeda, Y. Regulation of protein loading on poly(trimethylene carbonate), poly(l-lactic acid), and their copolymer: Effect of surface enrichment by polymer crystallinity. Mater. Sci. Eng. C 2012, 32, 988–993. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Liu, G.; Liu, X.; Jin, Q.; Ji, J. Self-Assembly of Near-Monodisperse Redox-Sensitive Micelles from Cholesterol-Conjugated Biomimetic Copolymers: Self-Assembly of Near-Monodisperse Redox-Sensitive Micelles. Macromol. Biosci. 2013, 13, 1084–1091. [Google Scholar] [CrossRef]
- Restani, R.B.; Pires, R.F.; Baptista, P.V.; Fernandes, A.R.; Casimiro, T.; Bonifácio, V.D.B.; Aguiar-Ricardo, A. Nano-in-Micro Sildenafil Dry Powder Formulations for the Treatment of Pulmonary Arterial Hypertension Disorders: The Synergic Effect of POxylated Polyurea Dendrimers, PLGA, and Cholesterol. Part. Part. Syst. Charact. 2020, 37, 1900447. [Google Scholar] [CrossRef]
- Edlund, U.; Albertsson, A.-C. Degradable Polymer Microspheres for Controlled Drug Delivery. In Degradable Aliphatic Polyesters; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2002; Volume 157, pp. 67–112. ISBN 978-3-540-42249-5. [Google Scholar]
- Zhang, R.; Qin, X.; Kong, F.; Chen, P.; Pan, G. Improving cellular uptake of therapeutic entities through interaction with components of cell membrane. Drug Deliv. 2019, 26, 328–342. [Google Scholar] [CrossRef] [Green Version]
- Stewart, M.P.; Langer, R.; Jensen, K.F. Intracellular Delivery by Membrane Disruption: Mechanisms, Strategies, and Concepts. Chem. Rev. 2018, 118, 7409–7531. [Google Scholar] [CrossRef] [PubMed]
- Nes, W.D. Biosynthesis of Cholesterol and Other Sterols. Chem. Rev. 2011, 111, 6423–6451. [Google Scholar] [CrossRef]
- Sadava, D.E. (Ed.) Life: The Science of Biology, 9th ed.; Sinauer Associates; W. H. Freeman & Co.: Sunderland, MA, USA; Gordonsville, VA, USA, 2011; ISBN 978-1-4292-1962-4. [Google Scholar]
- Cerqueira, N.M.F.S.A.; Oliveira, E.F.; Gesto, D.S.; Santos-Martins, D.; Moreira, C.; Moorthy, H.N.; Ramos, M.J.; Fernandes, P.A. Cholesterol Biosynthesis: A Mechanistic Overview. Biochemistry 2016, 55, 5483–5506. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2012, 64, 302–315. [Google Scholar] [CrossRef]
- Albuquerque, H.; Santos, C.; Silva, A. Cholesterol-Based Compounds: Recent Advances in Synthesis and Applications. Molecules 2018, 24, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morzycki, J.W. Recent advances in cholesterol chemistry. Steroids 2014, 83, 62–79. [Google Scholar] [CrossRef]
- Zhou, Y.; Briand, V.; Sharma, N.; Ahn, S.; Kasi, R. Polymers Comprising Cholesterol: Synthesis, Self-Assembly, and Applications. Materials 2009, 2, 636–660. [Google Scholar] [CrossRef]
- Misiak, P.; Niemirowicz-Laskowska, K.; Markiewicz, K.H.; Misztalewska-Turkowicz, I.; Wielgat, P.; Kurowska, I.; Siemiaszko, G.; Destarac, M.; Car, H.; Wilczewska, A.Z. Evaluation of Cytotoxic Effect of Cholesterol End-Capped Poly(N-Isopropylacrylamide)s on Selected Normal and Neoplastic Cells. Int. J. Nanomedicine 2020, 15, 7263–7278. [Google Scholar] [CrossRef]
- Wang, J.-S.; Matyjaszewski, K. Controlled/“living” radical polymerization. atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Kato, M.; Kamigaito, M.; Sawamoto, M.; Higashimura, T. Polymerization of Methyl Methacrylate with the Carbon Tetrachloride/Dichlorotris- (triphenylphosphine)ruthenium(II)/Methylaluminum Bis(2,6-di-tert-butylphenoxide) Initiating System: Possibility of Living Radical Polymerization. Macromolecules 1995, 28, 1721–1723. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K. (Bill); Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living Free-Radical Polymerization by Reversible Addition−Fragmentation Chain Transfer: The RAFT Process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Moad, G. RAFT polymerization to form stimuli-responsive polymers. Polym. Chem. 2017, 8, 177–219. [Google Scholar] [CrossRef]
- Messina, M.S.; Messina, K.M.M.; Bhattacharya, A.; Montgomery, H.R.; Maynard, H.D. Preparation of biomolecule-polymer conjugates by grafting-from using ATRP, RAFT, or ROMP. Prog. Polym. Sci. 2020, 100, 101186. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Glaria, A.; Beija, M.; Bordes, R.; Destarac, M.; Marty, J.-D. Understanding the Role of ω-End Groups and Molecular Weight in the Interaction of PNIPAM with Gold Surfaces. Chem. Mater. 2013, 25, 1868–1876. [Google Scholar] [CrossRef]
- Zou, T.; Li, F.; Cheng, S.-X.; Zhuo, R.-X. Synthesis and characterization of end-capped biodegradable oligo/poly(trimethylene carbonate)s. J. Biomater. Sci. Polym. Ed. 2006, 17, 1093–1106. [Google Scholar] [CrossRef]
- Hong, B.J.; Chipre, A.J.; Nguyen, S.T. Acid-Degradable Polymer-Caged Lipoplex (PCL) Platform for siRNA Delivery: Facile Cellular Triggered Release of siRNA. J. Am. Chem. Soc. 2013, 135, 17655–17658. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-M.; Chen, H.; Dettmer, C.M.; O’Halloran, T.V.; Nguyen, S.T. Polymer-Caged Lipsomes: A pH-Responsive Delivery System with High Stability. J. Am. Chem. Soc. 2007, 129, 15096–15097. [Google Scholar] [CrossRef]
- Alves, P.; Hugo, A.A.; Tymczyszyn, E.E.; Ferreira, A.F.; Fausto, R.; Pérez, P.F.; Coelho, J.F.J.; Simões, P.N.; Gómez-Zavaglia, A. Effect of cholesterol-poly(N,N-dimethylaminoethyl methacrylate) on the properties of stimuli-responsive polymer liposome complexes. Colloids Surf. B Biointerfaces 2013, 104, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Simões, M.G.; Alves, P.; Carvalheiro, M.; Simões, P.N. Stability effect of cholesterol-poly(acrylic acid) in a stimuli-responsive polymer-liposome complex obtained from soybean lecithin for controlled drug delivery. Colloids Surf. B Biointerfaces 2017, 152, 103–113. [Google Scholar] [CrossRef]
- Simões, M.G.; Hugo, A.; Alves, P.; Pérez, P.F.; Gómez-Zavaglia, A.; Simões, P.N. Long term stability and interaction with epithelial cells of freeze-dried pH-responsive liposomes functionalized with cholesterol-poly(acrylic acid). Colloids Surf. B Biointerfaces 2018, 164, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Szymanowski, F.; Hugo, A.A.; Alves, P.; Simões, P.N.; Gómez-Zavaglia, A.; Pérez, P.F. Endocytosis and intracellular traffic of cholesterol-PDMAEMA liposome complexes in human epithelial-like cells. Colloids Surf. B Biointerfaces 2017, 156, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-P.; Ji, J.; Chen, W.-D.; Shen, J.-C. Novel biomimetic polymersomes as polymer therapeutics for drug delivery. J. Control. Release 2005, 107, 502–512. [Google Scholar] [CrossRef]
- Xu, J.-P.; Ji, J.; Chen, W.-D.; Shen, J.-C. Novel Biomimetic Surfactant: Synthesis and Micellar Characteristics. Macromol. Biosci. 2005, 5, 164–171. [Google Scholar] [CrossRef]
- Xu, J.P.; Ji, J.; Chen, W.-D.; Shen, J.C. Biomimetic Amphiphiles for Polymeric Micellar Carrier System. Key Eng. Mater. 2005, 288–289, 465–468. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.-R.; Jiang, X.-S.; Cheng, S.-X.; Zhuo, R.-X. Studies on functionalization of poly(ε-caprolactone) by a cholesteryl moiety. J. Biomater. Sci. Polym. Ed. 2005, 16, 1095–1108. [Google Scholar] [CrossRef]
- Blasco, E.; Sims, M.B.; Goldmann, A.S.; Sumerlin, B.S.; Barner-Kowollik, C. 50th Anniversary Perspective: Polymer Functionalization. Macromolecules 2017, 50, 5215–5252. [Google Scholar] [CrossRef]
- Gauthier, M.A.; Gibson, M.I.; Klok, H.-A. Synthesis of Functional Polymers by Post-Polymerization Modification. Angew. Chem. Int. Ed. 2009, 48, 48–58. [Google Scholar] [CrossRef]
- Tian, Q.; Shi, J.; Zhao, X.; Di, D.; Deng, Y.; Song, Y. The antitumor efficacy of docetaxel is enhanced by encapsulation in novel amphiphilic polymer cholesterol-coupled tocopheryl polyethylene glycol 1000 succinate micelles. Drug Deliv. Transl. Res. 2017, 7, 642–653. [Google Scholar] [CrossRef]
- Varshosaz, J.; Taymouri, S.; Hassanzadeh, F.; Haghjooy Javanmard, S.; Rostami, M. Folated Synperonic-Cholesteryl Hemisuccinate Polymeric Micelles for the Targeted Delivery of Docetaxel in Melanoma. BioMed Res. Int. 2015, 2015, 1–17. [Google Scholar] [CrossRef]
- Tian, Y.; Mi, G.; Chen, Q.; Chaurasiya, B.; Li, Y.; Shi, D.; Zhang, Y.; Webster, T.J.; Sun, C.; Shen, Y. Acid-Induced Activated Cell-Penetrating Peptide-Modified Cholesterol-Conjugated Polyoxyethylene Sorbitol Oleate Mixed Micelles for pH-Triggered Drug Release and Efficient Brain Tumor Targeting Based on a Charge Reversal Mechanism. ACS Appl. Mater. Interfaces 2018, 10, 43411–43428. [Google Scholar] [CrossRef]
- Deng, Y.; Song, Y.; Tian, Q.; Huang, Z.; Fan, D.; She, Z.; Liu, X.; Cheng, X.; Yu, B. Self-assembled micelles of novel amphiphilic copolymer cholesterol-coupled F68 containing cabazitaxel as a drug delivery system. Int. J. Nanomed. 2014, 2307. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-M.; Yang, Y.-Y.; Leong, K.W. Thermally responsive polymeric micellar nanoparticles self-assembled from cholesteryl end-capped random poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide): Synthesis, temperature-sensitivity, and morphologies. J. Colloid Interface Sci. 2003, 266, 295–303. [Google Scholar] [CrossRef]
- Angarita, A.V.; Umaña-Perez, A.; Perez, L.D. Enhancing the performance of PEG-b-PCL-based nanocarriers for curcumin through its conjugation with lipophilic biomolecules. J. Bioact. Compat. Polym. 2020, 35, 399–413. [Google Scholar] [CrossRef]
- Badwaik, V.D.; Aicart, E.; Mondjinou, Y.A.; Johnson, M.A.; Bowman, V.D.; Thompson, D.H. Structure-property relationship for in vitro siRNA delivery performance of cationic 2-hydroxypropyl-β-cyclodextrin: PEG-PPG-PEG polyrotaxane vectors. Biomaterials 2016, 84, 86–98. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Piao, C.; Oh, J.; Lee, M. Self-assembled polymeric micelles for combined delivery of anti-inflammatory gene and drug to the lungs by inhalation. Nanoscale 2018, 10, 8503–8514. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Zhao, W.-Y.; Tsai, H.-C.; Hsu, W.-H.; Lo, C.-L.; Hsiue, G.-H. Sterically Polymer-Based Liposomal Complexes with Dual-Shell Structure for Enhancing the siRNA Delivery. Biomacromolecules 2012, 13, 664–675. [Google Scholar] [CrossRef]
- Basel, M.T.; Shrestha, T.B.; Troyer, D.L.; Bossmann, S.H. Protease-Sensitive, Polymer-Caged Liposomes: A Method for Making Highly Targeted Liposomes Using Triggered Release. ACS Nano 2011, 5, 2162–2175. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, L.; Tian, Y.; Ji, X.; Hu, Q.; Zhou, B.; Zhenyu, D.; Heng, X.; Yang, L. Cholesterol-modified DP7 enhances the effect of individualized cancer immunotherapy based on neoantigens. Biomaterials 2020, 241, 119852. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Zhuang, D.; Yang, J.; Yang, J. Bionanoparticles of amphiphilic copolymers polyacrylate bearing cholesterol and ascorbate for drug delivery. J. Colloid Interface Sci. 2012, 377, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Lynge, M.E.; Baekgaard Laursen, M.; Hosta-Rigau, L.; Jensen, B.E.B.; Ogaki, R.; Smith, A.A.A.; Zelikin, A.N.; Städler, B. Liposomes as Drug Deposits in Multilayered Polymer Films. ACS Appl. Mater. Interfaces 2013, 5, 2967–2975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lynge, M.E.; Nielsen, M.B.; Schattling, P.S.; Han, X.; Städler, B. Patterned Liposome-Polymer Composite Coatings. ChemNanoMat 2016, 2, 822–829. [Google Scholar] [CrossRef]
- Sevimli, S.; Inci, F.; Zareie, H.M.; Bulmus, V. Well-Defined Cholesterol Polymers with pH-Controlled Membrane Switching Activity. Biomacromolecules 2012, 13, 3064–3075. [Google Scholar] [CrossRef] [Green Version]
- Sevimli, S.; Sagnella, S.; Macmillan, A.; Whan, R.; Kavallaris, M.; Bulmus, V.; Davis, T.P. The endocytic pathway and therapeutic efficiency of doxorubicin conjugated cholesterol-derived polymers. Biomater. Sci. 2015, 3, 323–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Luo, T.; Sheng, R.; Li, H.; Sun, J.; Cao, A. Amphiphilic Diblock Terpolymer PMAgala-b-P(MAA-co-MAChol)s with Attached Galactose and Cholesterol Grafts and Their Intracellular pH-Responsive Doxorubicin Delivery. Biomacromolecules 2016, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Chen, J.; Yang, J.; Jiang, W.; Han, H.; Li, Q.; Yang, Y. Chemoenzymatic synthesis of a cholesterol-g-poly(amine-co-ester) carrier for p53 gene delivery to inhibit the proliferation and migration of tumor cells. New J. Chem. 2018, 42, 13541–13548. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, W.; Han, H.; Yang, J.; Chen, W.; Wang, Y.; Tang, J.; Li, Q. Chemoenzymatic Synthesis of Cholesterol-g-Poly(amine-co-ester) Amphiphilic Copolymer as a Carrier for miR-23b Delivery. ACS Macro Lett. 2017, 6, 523–528. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, D.; Li, J.; Xia, D.; Yu, M.; Tao, J.; Zhang, X.; Li, L.; Gan, Y. NPC1L1-Targeted Cholesterol-Grafted Poly(β-Amino Ester)/pDNA Complexes for Oral Gene Delivery. Adv. Healthc. Mater. 2019, 8, 1800934. [Google Scholar] [CrossRef]
- Valencia-Serna, J.; Kucharski, C.; Chen, M.; Kc, R.; Jiang, X.; Brandwein, J.; Uludağ, H. siRNA-mediated BCR-ABL silencing in primary chronic myeloid leukemia cells using lipopolymers. J. Control. Release 2019, 310, 141–154. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Fang, J.-Y.; Wang, S.-W.; Lee, R.-S. Synthesis and characterization of triple-responsive PNiPAAm-S-S-P(αN3CL-g-alkyne) copolymers bearing cholesterol and fluorescence monitor. React. Funct. Polym. 2018, 130, 29–42. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Z.; Chen, H.; Gao, J.; Liang, W. Transfection efficiency and intracellular fate of polycation liposomes combined with protamine. Biomaterials 2011, 32, 1412–1418. [Google Scholar] [CrossRef]
- Ding, K.; Li, R.; Ma, Y.; Li, N.; Zhang, T.; Cheng-Mei, X.; Jiang, H.-T.; Gong, Y.-K. Folate Ligand Orientation Optimized during Cell Membrane Mimetic Micelle Formation for Enhanced Tumor Cell Targeting. Langmuir 2019, 35, 1257–1265. [Google Scholar] [CrossRef]
- Jiang, H.-T.; Ding, K.; Meng, F.-N.; Bao, L.-L.; Chai, Y.-D.; Gong, Y.-K. Anti-phagocytosis and tumor cell targeting micelles prepared from multifunctional cell membrane mimetic polymers. J. Mater. Chem. B 2016, 4, 5464–5474. [Google Scholar] [CrossRef]
- Remant, K.; Thapa, B.; Valencia-Serna, J.; Domun, S.S.; Dimitroff, C.; Jiang, X.; Uludağ, H. Cholesterol grafted cationic lipopolymers: Potential siRNA carriers for selective chronic myeloid leukemia therapy. J. Biomed. Mater. Res. A 2020, 108, 565–580. [Google Scholar] [CrossRef]
- Kc, R.; Thapa, B.; Ubeda, A.; Jiang, X.; Uludağ, H. BCR-Abl Silencing by siRNA: A Potent Approach to Sensitize Chronic Myeloid Leukemia Cells to Tyrosine Kinase Inhibitor Therapy. Stem Cells Dev. 2019, 28, 734–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, H.; Shin-Ya, M.; Takeda, S.; Hashimoto, Y.; Mukai, S.; Sawada, S.; Adachi, T.; Akiyoshi, K.; Miki, T.; Mazda, O. Cycloamylose-nanogel drug delivery system-mediated intratumor silencing of the vascular endothelial growth factor regulates neovascularization in tumor microenvironment. Cancer Sci. 2014, 105, 1616–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.; Luo, X.; Wu, H.; Yu, F.; Wang, K.; Sun, M.; Oupicky, D. Cholesterol Modification Enhances Antimetastatic Activity and siRNA Delivery Efficacy of Poly(ethylenimine)-Based CXCR4 Antagonists. Macromol. Biosci. 2018, 18, 1800234. [Google Scholar] [CrossRef]
- Sun, Y.-D.; Zhu, Y.-X.; Zhang, X.; Jia, H.-R.; Xia, Y.; Wu, F.-G. Role of Cholesterol Conjugation in the Antibacterial Photodynamic Therapy of Branched Polyethylenimine-Containing Nanoagents. Langmuir 2019, 35, 14324–14331. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xie, X.; Xu, X.; Zhang, L.; Zhou, X.; Yu, H.; Wu, P.; Wang, T.; Che, X.; Hu, Z. Development of dual ligand-targeted polymeric micelles as drug carriers for cancer therapy in vitro and in vivo. J. Mater. Chem. B 2014, 2, 2114. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Luo, X.; Wu, H.; Zhang, Q.; Wang, K.; Sun, M.; Oupicky, D. Combined Hydrophobization of Polyethylenimine with Cholesterol and Perfluorobutyrate Improves siRNA Delivery. Bioconjug. Chem. 2020, 31, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Chen, Y.; Oupický, D. Balancing polymer hydrophobicity for ligand presentation and siRNA delivery in dual function CXCR4 inhibiting polyplexes. Biomater. Sci. 2015, 3, 1114–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, N.; Hirano, S.; Takahashi, H.; Loethen, S.; Thompson, D.H.; Akiyoshi, K. Self-Assembled pH-Sensitive Cholesteryl Pullulan Nanogel As a Protein Delivery Vehicle. Biomacromolecules 2013, 14, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, J.; Liu, T. Fabrication of Mixed Polymeric Micelles Based on Stimuli-Responsive Amphiphilic Copolymers for Drug Delivery and Controlled Release. Nano 2020, 15, 2050040. [Google Scholar] [CrossRef]
- Chen, C.-J.; Wang, J.-C.; Zhao, E.-Y.; Gao, L.-Y.; Feng, Q.; Liu, X.-Y.; Zhao, Z.-X.; Ma, X.-F.; Hou, W.-J.; Zhang, L.-R.; et al. Self-assembly cationic nanoparticles based on cholesterol-grafted bioreducible poly(amidoamine) for siRNA delivery. Biomaterials 2013, 34, 5303–5316. [Google Scholar] [CrossRef]
- Gao, L.-Y.; Liu, X.-Y.; Chen, C.-J.; Wang, J.-C.; Feng, Q.; Yu, M.-Z.; Ma, X.-F.; Pei, X.-W.; Niu, Y.-J.; Qiu, C.; et al. Core-Shell type lipid/rPAA-Chol polymer hybrid nanoparticles for in vivo siRNA delivery. Biomaterials 2014, 35, 2066–2078. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Q.; Hu, Q.; Li, Y.; Tang, G.; Chu, P.K. Restoration of chemosensitivity by multifunctional micelles mediated by P-gp siRNA to reverse MDR. Biomaterials 2014, 35, 8621–8634. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.O.; Nagarsenker, M.S.; Barhate, C.R.; Padhye, S.G.; Dhawan, V.V.; Bhattacharyya, D.; Viswanathan, C.L.; Steiniger, F.; Fahr, A. Cholesterol anchored arabinogalactan for asialoglycoprotein receptor targeting: Synthesis, characterization, and proof of concept of hepatospecific delivery. Carbohydr. Res. 2015, 408, 33–43. [Google Scholar] [CrossRef]
- Sawada, S.; Yukawa, H.; Takeda, S.; Sasaki, Y.; Akiyoshi, K. Self-assembled nanogel of cholesterol-bearing xyloglucan as a drug delivery nanocarrier. J. Biomater. Sci. Polym. Ed. 2017, 28, 1183–1198. [Google Scholar] [CrossRef]
- Shaki, H.; Ganji, F.; Kempen, P.J.; Dolatshahi-Pirouz, A.; Vasheghani-Farahani, E. Self-assembled amphiphilic-dextran nanomicelles for delivery of rapamycin. J. Drug Deliv. Sci. Technol. 2018, 44, 333–341. [Google Scholar] [CrossRef]
- Yao, X.; Chen, L.; Chen, X.; He, C.; Zheng, H.; Chen, X. Intercellular pH-responsive histidine modified dextran-g-cholesterol micelle for anticancer drug delivery. Colloids Surf. B Biointerfaces 2014, 121, 36–43. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Feng, Y.; Yu, G.; Zhou, Q.; He, F.; Xiao, D.; Chen, K.; Zhang, L. Self-aggregation behavior of hydrophobic sodium alginate derivatives in aqueous solution and their application in the nanoencapsulation of acetamiprid. Int. J. Biol. Macromol. 2018, 106, 418–424. [Google Scholar] [CrossRef]
- Liu, X.-M.; Pramoda, K.P.; Yang, Y.-Y.; Chow, S.Y.; He, C. Cholesteryl-grafted functional amphiphilic poly(N-isopropylacrylamide-co-N-hydroxylmethylacrylamide): Synthesis, temperature-sensitivity, self-assembly and encapsulation of a hydrophobic agent. Biomaterials 2004, 25, 2619–2628. [Google Scholar] [CrossRef]
- Pourmoazzen, Z.; Sadeghifar, H.; Chen, J.; Yang, G.; Zhang, K.; Lucia, L. The morphology, self-assembly, and host-guest properties of cellulose nanocrystals surface grafted with cholesterol. Carbohydr. Polym. 2020, 233, 115840. [Google Scholar] [CrossRef]
- Xu, Y.; Zi, Y.; Lei, J.; Mo, X.; Shao, Z.; Wu, Y.; Tian, Y.; Li, D.; Mu, C. pH-Responsive nanoparticles based on cholesterol/imidazole modified oxidized-starch for targeted anticancer drug delivery. Carbohydr. Polym. 2020, 233, 115858. [Google Scholar] [CrossRef]
- Devaraj, N.K. The Future of Bioorthogonal Chemistry. ACS Cent. Sci. 2018, 4, 952–959. [Google Scholar] [CrossRef] [Green Version]
- Misztalewska-Turkowicz, I.; Markiewicz, K.H.; Michalak, M.; Wilczewska, A.Z. NHC-copper complexes immobilized on magnetic nanoparticles: Synthesis and catalytic activity in the CuAAC reactions. J. Catal. 2018, 362, 46–54. [Google Scholar] [CrossRef]
- Cho, H.K.; Cheong, I.W.; Lee, J.M.; Kim, J.H. Polymeric nanoparticles, micelles and polymersomes from amphiphilic block copolymer. Korean J. Chem. Eng. 2010, 27, 731–740. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Baby, T.; Chen, D.; Weitz, D.A.; Zhao, C. Stable Polymer Nanoparticles with Exceptionally High Drug Loading by Sequential Nanoprecipitation. Angew. Chem. Int. Ed. 2020, 59, 4720–4728. [Google Scholar] [CrossRef]
- Allen, C.; Maysinger, D.; Eisenberg, A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf. B Biointerfaces 1999, 16, 3–27. [Google Scholar] [CrossRef]
- Mabrouk, E.; Cuvelier, D.; Pontani, L.-L.; Xu, B.; Lévy, D.; Keller, P.; Brochard-Wyart, F.; Nassoy, P.; Li, M.-H. Formation and material properties of giant liquid crystal polymersomes. Soft Matter 2009, 5, 1870. [Google Scholar] [CrossRef]
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Zhou, S.; Li, J.; Wang, J.; Chi, D.; Wang, X.; Lin, G.; He, Z.; Wang, Y. Remote loading paclitaxel–doxorubicin prodrug into liposomes for cancer combination therapy. Acta Pharm. Sin. B 2020, 10, 1730–1740. [Google Scholar] [CrossRef]
- Liu, A.; Wang, H.; Hou, X.; Ma, Y.; Yang, G.; Hou, Y.; Ding, Y. Combinatory antitumor therapy by cascade targeting of a single drug. Acta Pharm. Sin. B 2020, 10, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Scopel, R.; Falcão, M.A.; Cappellari, A.R.; Morrone, F.B.; Guterres, S.S.; Cassel, E.; Kasko, A.M.; Vargas, R.M.F. Lipid-polymer hybrid nanoparticles as a targeted drug delivery system for melanoma treatment. Int. J. Polym. Mater. Polym. Biomater. 2020, 1–12. [Google Scholar] [CrossRef]
- Krstić, M.; Manić, L.; Martić, N.; Vasiljević, D.; Mračević, S.Đ.; Vukmirović, S.; Rašković, A. Binary polymeric amorphous carvedilol solid dispersions: In vitro and in vivo characterization. Eur. J. Pharm. Sci. 2020, 150, 105343. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Lv, W.; Li, Y.; Chang, J.; Zhang, W.; Liu, C.; Sun, J. Improving Tumor Targeting of Exosomal Membrane-Coated Polymeric Nanoparticles by Conjugation with Aptamers. ACS Appl. Biol. Mater. 2020, 3, 2666–2673. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Siegwart, D.J.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Washburn, N.R.; Matyjaszewski, K. Nanostructured hybrid hydrogels prepared by a combination of atom transfer radical polymerization and free radical polymerization. Biomaterials 2009, 30, 5270–5278. [Google Scholar] [CrossRef] [Green Version]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Mok, H.; Lee, S.; Oh, Y.-K.; Park, T.G. Target-specific intracellular delivery of siRNA using degradable hyaluronic acid nanogels. J. Control. Release 2007, 119, 245–252. [Google Scholar] [CrossRef]
- Hayashi, H.; Iijima, M.; Kataoka, K.; Nagasaki, Y. pH-Sensitive Nanogel Possessing Reactive PEG Tethered Chains on the Surface. Macromolecules 2004, 37, 5389–5396. [Google Scholar] [CrossRef]
- Warren, D.S.; Sutherland, S.P.H.; Kao, J.Y.; Weal, G.R.; Mackay, S.M. The Preparation and Simple Analysis of a Clay Nanoparticle Composite Hydrogel. J. Chem. Educ. 2017, 94, 1772–1779. [Google Scholar] [CrossRef]
- D’Andrea, C.; Pezzoli, D.; Malloggi, C.; Candeo, A.; Capelli, G.; Bassi, A.; Volonterio, A.; Taroni, P.; Candiani, G. The study of polyplex formation and stability by time-resolved fluorescence spectroscopy of SYBR Green I-stained DNA. Photochem. Photobiol. Sci. 2014, 13, 1680–1689. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Tai, W. Insight into the siRNA transmembrane delivery—From cholesterol conjugating to tagging. WIREs Nanomed. Nanobiotechnol. 2020, 12. [Google Scholar] [CrossRef]







| Polymer | Form of Carrier | Drug or Dye | Mn or Mw (kDa) (Ð) | LE (%) | LC (%) | Lit. |
|---|---|---|---|---|---|---|
| Free Radical Polymerization (FRP) | ||||||
| Chol-pHPMAlac (mono:di = 30:70) | liposome | DOX | 10.5 (1.60–1.70) | 93.0 | N/A | [24] |
| Chol-pHPMAlac (mono:di = 44:56) | 10.0 (1.60–1.70) | 99.0 | ||||
| Chol-pHPMAlac (mono:di = 54:46) | 11.0 (1.60–1.70) | 100 | ||||
| Chol-pHPMAlac (mono:di = 67:33) | 11.0 (1.60–1.70) | 100 | ||||
| Nitroxide-Mediated Controlled Radical Polymerization (NMP) | ||||||
| Chol-PAA | PCLp | siRNA | N/A | 46.0 | 0.8 | [100,101] |
| Atom Transfer Radical Polymerization (ATRP) | ||||||
| Chol-PDMAEMA | liposome | CF | 5.4 (1.17) | N/A | N/A | [102] |
| calcein | ||||||
| Chol-PAA 5% in lip | liposome | calcein | 7.2 (N/A) | 29.6 | N/A | [103,104] |
| Chol-PAA 10% in lip | 46.1 | |||||
| Chol-PAA 20% in lip | 28.8 | |||||
| Chol-PAA 10% in lip crosslinked | 24.7 | |||||
| Chol-LC-PDMAEMA | liposome | calcein | N/A | N/A | N/A | [105] |
| Chol-PLA-SS-PMPC | micelle | Nile red | N/A | N/A | N/A | [79] |
| Chol-b-pMPC | polymersome | ADR | 6.4 (N/A) | 3.6 | N/A | [106] |
| 9.5 (N/A) | 4.2 | |||||
| 15.4 (N/A) | 4.0 | |||||
| micelle | ADR | 3.0 (N/A) | N/A | N/A | [107] | |
| 6.4 (N/A) | ||||||
| N/A | ||||||
| Chol-PEO | micelle | ADR | 1.7 (1.13) | N/A | 10.1 | [108] |
| 2.3 (1.10) | 16.2 | |||||
| 2.8 (1.10) | 16.9 | |||||
| Chol-PEGMA50 | micelle | QC | 33.2 (1.25) | N/A | 15.6 | [54] |
| Chol-PEGMA100 | 52.2 (1.32) | 14.1 | ||||
| Chol-PEGMA200 | 89.1 (1.55) | 14.1 | ||||
| Reversible Addition–Fragmentation Chain Transfer Polymerization (RAFT) | ||||||
| Chol-PNIPAAm | micelle | N/A | 3.2 (1.27) | N/A | N/A | [91] |
| 5.7 (1.35) | ||||||
| 6.1 (1.51) | ||||||
| 8.4 (1.64) | ||||||
| 10.9 (1.90) | ||||||
| Ring-Opening Polymerization (ROP) | ||||||
| Chol-PCL (nChol:nPCL = 1:4) | nanoparticle | prednisone acetate | 2.0 (1.49) | N/A | N/A | [109] |
| Chol-PCL (nChol:nPCL = 1:10) | 5.5 (1.34) | |||||
| Chol-PCL (nChol:nPCL = 1:20) | 7.2 (1.55) | |||||
| Chol-PCL (nChol:nPCL = 1:40) | 11.4 (1.69) | |||||
| Chol-PCL (nChol:nPCL = 1:80) | 16.2 (1.79) | |||||
| Chol-pTMC (nChol:nTMC = 1:4) | nanoparticle | prednisone acetate | 1.8 (1.26) | N/A | N/A | [99] |
| Chol-pTMC (nChol:nTMC = 1:10) | 2.7 (1.75) | |||||
| Chol-pTMC (nChol:nTMC = 1:20) | 5.2 (1.78) | |||||
| Chol-pTMC (nChol:nTMC = 1:40) | 9.7 (1.65) | 61.7 | 9.1 | |||
| Chol-pTMC (nChol:nTMC = 1:80) | 13.9 (1.80) | N/A | N/A | |||
| Organocatalytic Ring-Opening Polymerization (OC-ROP) | ||||||
| Chol-PTMC-PEG | nanoparticle | DOX | 6.6 (N/A) | N/A | 7.3 | [52] |
| Chol-PTMC | surface | FITC-BSA | 11.3 (1.20) | N/A | N/A | [78] |
| Chol-PTMC-PLA | 10.1 (1.40) | |||||
| Chol-PTMC-PMBC | 2.5 (1.20) | |||||
| Chol-PMBC | 3.3 (1.50) | |||||
| Polymer | Form of Carrier | Drug or Dye | Mn or Mw (kDa) (Ð) | LE (%) | LC (%) | Lit. |
|---|---|---|---|---|---|---|
| Esterification | ||||||
| Acetylene-PEG10K-G4-Chol16 | micelle | DOX | 14.2 (1.11) | N/A | 18.8 | [55] |
| TPL | 8.4 | |||||
| DOX + TPL | N/A/6.5 | |||||
| Rhodamine-PEG10K-G4-Chol16 | DOX | 17.6 (1.14) | N/A | 10.0 | ||
| mPEG-Chol | micelle | DTXL | N/A | 97.6 | 4.8 | [56] |
| mPEG-Chol/RGD-mPEG-Chol (10% mPEG-Chol w/w) | liposome | PTX (2.5% w/w) | N/A | 99.8 | 0.05 | [57] |
| PTX (5% w/w) | 99.6 | 0.08 | ||||
| PTX (7.5% w/w) | 99.1 | 1.15 | ||||
| PTX (10% w/w) | 97.3 | 1.62 | ||||
| mPEG-Chol/RGD-mPEG-Chol (20% mPEG-Chol w/w) | PTX (2.5% w/w) | 99.7 | 0.53 | |||
| PTX (5% w/w) | 99.1 | 0.81 | ||||
| PTX (7.5% w/w) | 98.9 | 1.06 | ||||
| PTX (10% w/w) | 95.1 | 1.48 | ||||
| mPEG-Chol | micelle | AmB | 5.9 (1.04) | 42.0 | 8.8 | [58] |
| mPEG-b-PCL-Chol | 10.1 (1.20) | 60.0 | 12.5 | |||
| TPGS-Chol | micelle | DTXL | N/A | 99.2 | 3.2 | [112] |
| Chol-PEG-GA | liposome | brucine | N/A | 82.5 | N/A | [59] |
| Chol-PEG2K/(γ-PGA-g-PLGA) | nanoparticle | DOX | N/A | 63.8 | 4.6 | [53] |
| Chol-PEG5K/(γ-PGA-g-PLGA) | 66.8 | 4.8 | ||||
| Chol-PEG10K/(γ-PGA-g-PLGA) | 66.6 | 4.7 | ||||
| Chol-PEG2K/(γ-PGA-g-PLGA) | ICG | 86.8 | 6.2 | |||
| Chol-PEG5K/(γ-PGA-g-PLGA) | 86.8 | 6.2 | ||||
| Chol-PEG10K/(γ-PGA-g-PLGA) | 84.9 | 6.1 | ||||
| PF127-Chol | micelle | DTXL (temp., ratio, solvent) | N/A | 81.0 | N/A | [113] |
| FA-PF127-Chol | 65.4–103.2 | |||||
| Chol-PSO | micelle | PTX | 2.4 | 80.1 | 18.6 | [114] |
| Chol-PSO-(HE)5-Fmoc/Chol-PSO-(RG)5-Pbf | N/A | 78.5 | 17.1 | |||
| F68-Chol | micelle | CABA | N/A | 98.1 | 3.2 | [115] |
| mPEG-Chol | micelle | QC | N/A | 93.5 | 3.7 | [60] |
| Biotin-PAE-g-mPEG-Chol | micelle | DOX | 11.8 (1.60) | 61.0 | 5.5 | [61] |
| PAE-g-mPEG-Chol | N/A | 47.0 | 4.2 | |||
| mPEG–PLA-Chol | micelle | CUR | N/A | 93.7 | 11.9 | [28] |
| PEG-PLLA-Chol | micelle | DOX | N/A | 45.3 | 8.3 | [62,63] |
| PEG-PDLA-Chol | 48.2 | 8.8 | ||||
| Chol-PEG | micelle | PTX | N/A | >90 | N/A | [64] |
| Chol–PEG–DUP1 | micelle | PTX | N/A | 96.4 | 24.9 | [65] |
| Chol-mPEG-RGD/mPEG-PLGA | nanoparticle | CUR (2% w/w) | N/A | 100 | 2.00 | [29] |
| CUR (3% w/w) | 98.7 | 2.96 | ||||
| CUR (4% w/w) | 97.8 | 3.91 | ||||
| CUR (5% w/w) | 96.0 | 4.80 | ||||
| CUR (7% w/w) | 70.7 | 4.95 | ||||
| P(NIPAAm-co-DMAAm)-g-Chol | micelle | Py | 2.9 (1.20) | N/A | 0.8 mg/g | [116] |
| P(NIPAAm-co-DMAAm)-g-Chol | 6.4 (1.30) | 1 mg/g | ||||
| Chol-PEG-TPP | liposome | CF | N/A | 1.8 | N/A | [66] |
| mPEG-b-PCL-Chol | micelle | CUR | 6.6 (1.17) | 32.0 | 8.8 | [117] |
| Amidation | ||||||
| Chol−PEG−PpIX | micelle anchored to liposome | itself | N/A | N/A | N/A | [67] |
| HA–SA–CYS–Chol | micelle | DTXL | 30.1 (1.70) | 89.7 | 4.8 | [16] |
| HA-Chol | nanoparticle | DTXL | N/A | 66.9 | 1.9 | [17] |
| TMX | 76.5 | 4.1 | ||||
| DTXL/TMX | 83.1/92.5 | 1.4/3.4 | ||||
| PEG-PAsp(DET)-Chol | micelle | pDNA | N/A | N/A | N/A | [68] |
| DMEDA-HPbCD-Chol:Pluronic F127 | polyplex | siRNA | N/A | N/A | N/A | [118] |
| DMEDA-HPbCD-Chol:Pluronic L81 | ||||||
| DMEDA-HPbCD-Chol:Pluronic L35 | ||||||
| PAMAM-Chol | micelle | RES | N/A | N/A | 46.5 | [119] |
| PEG-Chol-α-TOC | micelle | CUR (5% w/w) | N/A | 97.2 | 4.6 | [69] |
| CUR (10% w/w) | 98.4 | 8.4 | ||||
| CUR (15% w/w) | 98.6 | 14.2 | ||||
| CUR (20% w/w) | 74.3 | 15.2 | ||||
| mPEG-b-PEP-g-Chol) linear | micelle | DOX | 5.8 (1.45) | 42.1 | 15.7 | [70] |
| mPEG-b-PEP-g-Chol) Y-shape | 6.0 (1.33) | 50.4 | 20.2 | |||
| mPEG-b-PEP-g-Chol) Fork-shape | 6.5 (1.48) | 58.5 | 23.1 | |||
| mPEG-b-PAMAM-G1-Chol1 | micelle | DOX | 5.3 (N/A) | 40.0 | 4.7 | [71] |
| PTX | 5.4 | 0.7 | ||||
| DOX/PTX | 38.1/6.5 | 4.3/0.7 | ||||
| mPEG-b-PAMAM-G2-Chol2 | DOX | 5.9 (N/A) | 40.7 | 4.8 | ||
| PTX | 7.2 | 0.8 | ||||
| DOX/PTX | 38.4/6.9 | 4.2/0.8 | ||||
| mPEG-b-PAMAM-G4-Chol4 | DOX | 7.2 (N/A) | 40.2 | 4.8 | ||
| PTX | 8.3 | 1.0 | ||||
| DOX/PTX | 34.5/8.3 | 3.9/1.0 | ||||
| mPEG-b-PAMAM-G8-Chol8 | DOX | N/A | 40.1 | 4.7 | ||
| PTX | 18.2 | 2.2 | ||||
| DOX/PTX | 36.8/19.4 | 4.2/2.2 | ||||
| Chol-P(HEMA-Lys) | liposome | siRNA | N/A (1.20) | N/A | N/A | [120] |
| mPEG-P(HPMA-g-His)-Chol | liposome | DOX | 12.3 (1.06) | 81.3 | 18.2 | [25,26] |
| (Chol-PLGVRK-PEG):(DUPA-PEG-Chol) = 1:9 | micelle | CABA (25% w/w) | N/A | 79.7 | 12.0 | [72] |
| CABA (200% w/w) | 38.9 | 43.8 | ||||
| Chol-g-uPA-PAA | liposome | CF | N/A | N/A | N/A | [121] |
| CS-g-Chol-g-FA | micelle | PTX (4h dialysis) | N/A | 75.6 | 12.9 | [11] |
| PTX (8h dialysis) | 63.1 | 10.5 | ||||
| PTX (12h dialysis) | 56.5 | 7.4 | ||||
| PTX (24h dialysis) | 32.7 | 5.5 | ||||
| Chol-DP7 | micelle | itself | N/A | N/A | N/A | [122] |
| Radical Cross-Coupling | ||||||
| p(HPMA-r-NAS)-Chol | polyplex | siRNA | 17.7 (1.40) | N/A | N/A | [27] |
| p(HPMA-r-AEDA)-Chol | 24.7 (1.20) | |||||
| p(HPMA-DMAE-r-AEDA)-Chol | 34.1 (1.30) | |||||
| Hydrazone Formation | ||||||
| mPEG-Hz-Chol | liposome | Arctigenin | N/A | 93.8 | N/A | [73] |
| GEM | 2.6 (N/A) | 37.0 | 4.0 | [74,75] | ||
| Polymer | Form of Carrier | Drug or Dye | Mn or Mw (kDa) (Ð) | LE (%) | LC (%) | Lit. |
|---|---|---|---|---|---|---|
| Free Radical Polymerization (FRP) | ||||||
| mPEG-Chol-DMA (nChol:nDMA = 1:7) | polymersome | FITC-CM-Dex | N/A | 60.0 | N/A | [30] |
| mPEG-Chol-DMA (nChol:nDMA = 1:3) | 59.0 | |||||
| mPEG-Chol-DMA (nChol:nDMA = 1:1) | N/A | |||||
| mPEG-Chol-DMA (nChol:nDMA = 3:1) | ||||||
| mPEG-Chol | ||||||
| Atom Transfer Radical Polymerization (ATRP) | ||||||
| PEG-SS-PAECChol | polymersome | Calcein | 6.7 (1.14) | 68.0 | 5.5 | [35] |
| PEG-b-PAECChol | 6.0 (1.13) | 74.0 | 6.0 | |||
| Reversible Addition–Fragmentation Chain Transfer Polymerization (RAFT) | ||||||
| P(AChol15-co-mPEG5,110) | micelle | CPT | 39.0 (1.44) | N/A | 5.5 | [36] |
| P(AChol3-co-mPEG23,22) | 25.0 (1.26) | N/A | 3.5 | |||
| P(CholDEGA-b-(AAA-r-BnAAA)) (52% hydrogenated) | micelle | Nile red | N/A | 25.0 | N/A | [123] |
| P(CholDEGA-b-(AAA-r-BnAAA)) (70% hydrogenated) | 25.0 | N/A | ||||
| P(CholDEGA-b-(AAA-r-BnAAA)) (85% hydrogenated) | 5.0 | N/A | ||||
| P(CholDEGA-b-(AAA-r-BnAAA)) (52% hydrogenated) | IBU | >40 | >25 | |||
| P(CholDEGA-b-(AAA-r-BnAAA)) (70% hydrogenated) | >30 | >25 | ||||
| P(CholDEGA-b-(AAA-r-BnAAA)) (85% hydrogenated) | >15 | >10 | ||||
| PLL(PMA-co-MAChol) | liposome | PTX | 33.0 (1.05) | N/A | N/A | [124,125] |
| P(MAA-co-MAChol) (2 mol% chol) | nanocomplex | DOX | 16.5 (1.19) | N/A | N/A | [126,127] |
| P(MAA-co-MAChol) (4 mol% chol) | 15.8 (1.10) | |||||
| P(MAA-co-MAChol) (8 mol% chol) | 18.0 (1.11) | |||||
| P(MAgala18-b-MAChol14) | micelle | DOX | 12.8 (1.26) | 47.1 | 10.5 | [128] |
| P(MAgala18-b-(MAA5-co-MAChol14)) | N/A | 61.5 | 13.3 | |||
| P(MAgala18-b-(MAA16-co-MAChol12)) | 81.9 | 17.0 | ||||
| P(MAgala18-b-(MAA26-co-MAChol9)) | 91.2 | 18.6 | ||||
| P(HPMA-co-MA-εAhx-NHNH2-co-MA-εAhx-Chol) | nanoparticle | DOX | 50 (1.39) | N/A | 6.0 | [23] |
| Organocatalytic Ring-Opening Polymerization (OC-ROP) | ||||||
| mPEG113-b-P(MTC-Chol)4 | micelle | N/A | 7.5 (1.12) | N/A | N/A | [31] |
| mPEG113-b-P(MTC-Chol)11 | 11.8 (1.21) | |||||
| mPEG113-b-P(MTC-Chol11) | nanoparticle | PTX | 11.8 (1.21) | N/A | 3.8 | [32] |
| mPEG113-b-P(MTC-Chol8-co-TMC8) | 10.7 (1.18) | 9.2 | ||||
| mPEG113-b-P(MTC-Chol11-co-TMC30) | 14.8 (1.20) | 15.0 | ||||
| mPEG113-b-P(MTC-Chol18-co-TMC55) | 21.7 (1.17) | 8.4 | ||||
| Ring-Opening Metathesis Polymerization (ROMP) | ||||||
| P(NBChol-b-NBmPEG) | nanoparticle | DOX | 162 (1.30) | 58.0 | 14.5 | [33] |
| P(NBChol)50-b-(NBmPEG)170 | nanoparticle | DOX | 126 (1.24) | 88.4 | 22.1 | [34] |
| P(NBChol)75-b-(NBmPEG)255 | 216 (1.16) | 68.8 | 17.2 | |||
| P(NBChol)180-b-(NBmPEG)222 | 118 (1.16) | 79.2 | 19.8 | |||
| Polymer | Form of Carrier | Drug or Dye | Mn or Mw (kDa) (Ð) | LE (%) | LC (%) | Dg (%) | Lit. |
|---|---|---|---|---|---|---|---|
| Amidation | |||||||
| PEI-Chol | liposome | pDNA (pGL3 promoter) | N/A | N/A | N/A | N/A | [134] |
| HA-Chol | nanogel | rhGH/EPO lysozyme/exendin-4 | 52.0 (N/A) | N/A | N/A | 3.0 | [14] |
| 54.0 (N/A) | 7.0 | ||||||
| 59.0 (N/A) | 15.0 | ||||||
| 66.0 (N/A) | 27.0 | ||||||
| 75.0 (N/A) | 42.0 | ||||||
| P(MPC-co-NPEM)-g-Chol (nMPC:nChol = 86:14) | micelle | DOX | 11.2 (1.95) | 53.8 | 21.5 | 100 | [135] |
| P(MPC-co-NPEM)-g-Chol-g-FA (nMPC:nChol:nFA = 56:14:30) | 12.1 (2.16) | 53.5 | 21.4 | ||||
| P(MPC-co-NPEM)-g-Chol-g-FA (nMPC:nChol = 74:26) | micelle | DOX | 6.6 (N/A) | 48.6 | 19.6 | [136] | |
| P(MPC-co-NPEM)-g-Chol-g-FA (nMPC:nChol:nFA = 64:27:9) | N/A | 52.6 | 21.1 | ||||
| P(MPC-co-NPEM)-g-Chol-g-FA (nMPC:nChol:nFA = 61:16:23) | DOX (10% w/w) | 81.9 | 8.2 | ||||
| DOX (20% w/w) | 77.9 | 15.6 | |||||
| DOX (40% w/w) | 62.3 | 24.9 | |||||
| DOX (50% w/w) | 67.4 | 33.7 | |||||
| P(MPC-co-NPEM)-g-Chol-g-FA (nMPC:nChol:nFA = 58:11:31) | DOX | 11.4 (N/A) | 58.8 | 23.5 | |||
| γ-PGA-g-Chol | hydrogel | DOX | N/A | N/A | 6.39 | 96.2 | [76] |
| PEI-Chol | polyplex | siRNA | N/A | N/A | N/A | N/A | [137,138] |
| PEI-Chol (nPEI:nChol = 1:7.5) | micelle | SFB | 9.8 (N/A) | N/A | N/A | N/A | [37] |
| PEI-Chol (nPEI:nChol = 1:15.5) | 13.1 (N/A) | 13.1 | |||||
| PEI-Chol-PEG (nPEI:nChol:nPEG = 1:7.5:1) | 15.3 (N/A) | N/A | |||||
| PEI-Chol-PEG (nPEI:nChol:nPEG = 1:15.5:1) | 23.9 (N/A) | N/A | |||||
| Chol-CA-Spe | nanogel | siRNA | N/A | N/A | N/A | 3.1 | [139] |
| Cyc-PEI-Chol (nChol:nCyc = 0.17) | polyplex | siRNA | 28.8 (N/A) | N/A | N/A | N/A | [140] |
| Cyc-PEI-Chol (nChol:nCyc = 0.33) | 32.4 (N/A) | ||||||
| Cyc-PEI-Chol (nChol:nCyc = 0.53) | 36.9 (N/A) | ||||||
| PEI-Chol | nanoparticle | Ce6 | N/A | N/A | 35 | N/A | [141] |
| Chol-GC | micelle | DOX | N/A | 80.9 | 10.8 | 6.1 | [142] |
| Chol-GC-FA | 87.0 | 11.6 | |||||
| NLS-Chol-GC | 77.4 | 10.4 | |||||
| NLS-Chol-GC-FA | 79.0 | 10.6 | |||||
| Chol-GC | Cou6 | 89.8 | 1.76 | ||||
| Chol-GC-FA | 87.0 | 1.71 | |||||
| NLS-Chol-GC | 90.1 | 1.77 | |||||
| NLS-Chol-GC-FA | 89.6 | 1.73 | |||||
| PEI-Chol (nChol:nPEI = 25.8) | polyplex | siRNA | N/A | N/A | N/A | N/A | [143] |
| PEI-Chol (nChol:nPEI = 52.5) | |||||||
| PEI-Chol (nChol:nPEI = 102.44) | |||||||
| F-PEI-Chol (nChol:nF-PEI = 21.3) | |||||||
| F-PEI-Chol (nChol:nF-PEI = 50.9) | |||||||
| F-PEI-Chol (nChol:nF-PEI = 105.6) | |||||||
| PAMD-Chol (17% w/w of Chol) | polyplex | siRNA | 16.7 | N/A | N/A | N/A | [144] |
| PAMD-Chol (25% w/w of Chol) | 18.5 | ||||||
| PAMD-Chol (34% w/w of Chol) | 21.1 | ||||||
| PEI-Chol | N/A | siRNA | N/A | N/A | N/A | N/A | [132] |
| Click Reaction | |||||||
| PNIPAAm10-SS-P(αN3CL-g-CholPA)10 | micelle | IMC | 6.0 (1.24) | 82.8 | 40.4 | N/A | [133] |
| PNIPAAm10-SS-P(αN3CL10-g-PyrePA3/-CholPA7) | 5.7 (1.40) | 71.9 | 35.9 | ||||
| acL-Chol-PN | nanogel | FITC-BSA | 1 020 (N/A) | N/A | N/A | 1.7 | [145] |
| acS-Chol-PN | 1 130 (N/A) | 1.5 | |||||
| Hydrazone Formation | |||||||
| P(HPMA-co-MA-εAhx-NHNH2-co-MA-εAhx-Chol) | nanoparticle | DOX | 21.1 (1.65) | 98.0 | 1.7 | [19] | |
| DTXL | 95.0 | 5.5 | |||||
| P(HPMA-co-MA-εAhx-NHNH2-co-MA-εAhx-opBChol) | micelle | DOX | 38.0 (1.8) | N/A | 9.4 | [20] | |
| P(HPMA-co-MA-εAhx-NHNH2-co-MA-εAhx-Chol5α) | 24.5 (1.9) | 8.1 | |||||
| P(HPMA-co-MA-εAhx-NHNH2-co-MA-εAhx-Chol43) | 25.5 (1.8) | 8.2 | |||||
| P(HPMA-co-MA-εAhx-NHNH2-co-MA-εAhx-Chol5α) | micelle | DOX | 26.6 (1.88) | N/A | 8.2 | N/A | [21,22] |
| P(HPMA-co-MA-εAhx-NHNH2-co-MA-εAhx-opBChol) | 30.7 (1.65) | 11.2 | |||||
| P(HPMA-co-MA-εAhx-NHNH2-co-MA-εAhx-LevChol) | 28.5 (1.89) | 10.9 | |||||
| P(HPMA-co-MA-εAhx-NHNH2-co-MA-εAhx-Chol43) | 26.8 (1.72) | 7.9 | |||||
| Nucleophilic Substitution (Br to N) | |||||||
| Chol-g-P(MSC-PDL) | nanoparticle | plasmid p3XFLAG-CMV-p53 | 8.3 (1.95) | N/A | N/A | 9.7 | [129] |
| miR-23b | [130] | ||||||
| Chol-PHP | polyplex | pDNA | 12.5 (N/A) | N/A | N/A | 31.6 | [131] |
| Supercritical CO2-Assisted Spray Drying (SASD) | |||||||
| PURE-G4-OMeOx48[PLGA-Chol] | microparticle | SDF | N/A | N/A | 19.1 | N/A | [80] |
| PURE-G4-OEtOx48[PLGA-Chol] | 22.1 | ||||||
| Boronate Linkage | |||||||
| mPEG-PLL-g-DHPA/Chol-PBA (DHPA:Chol-PBA = 3:1) | nanoassembly | DOX | N/A | 8.1 | 2.0 | N/A | [38] |
| mPEG-PLL-g-DHPA/Chol-PBA (DHPA:Chol-PBA = 3:2) | 30.1 | 7.5 | |||||
| Esterification | |||||||
| PAE(-SS-mPEG)-g-Chol) | nanoparticle | DOX | 12.95 (1.45) | 55.4 | 10.8 | N/A | [39] |
| PAE(-SS-mPEG)-g-Chol)/PAE-g-mPEG-g-Chol/(mass ratio = 2:1) | micelle | DOX (10% w/w) | 12.95 (1.45)/8.79 (1.90) | 61.2 | 16.1 | N/A | [39,146] |
| DOX (20% w/w) | 64.7 | 26.4 | |||||
| DOX (30% w/w) | 55.7 | 28.8 | |||||
| PAE(-SS-mPEG)-g-Chol)/PAE-g-mPEG-g-Chol/(mass ratio = 1:1) | DOX (10% w/w) | 63.5 | 16.7 | ||||
| DOX (20% w/w) | 69.8 | 28.5 | |||||
| DOX (30% w/w) | 60.9 | 31.5 | |||||
| PAE(-SS-mPEG)-g-Chol)/PAE-g-mPEG-g-Chol/(mass ratio = 1:2) | DOX (10% w/w) | 59.1 | 15.8 | ||||
| DOX (20% w/w) | 63.0 | 25.7 | |||||
| DOX (30% w/w) | 53.9 | 27.9 | |||||
| poly(BAC-AMPD)-g-PEG-g-Chol | micelle | DOX | N/A | 27.1 | 5.4 | 54.5 | [40] |
| Chol-CS | nanoparticle | ATRA (10% w/w) | N/A | 88.7 | 8.0 | 4 | [12] |
| ATRA (20% w/w) | 82.3 | 11.8 | |||||
| ATRA (40% w/w) | 77.9 | 24.3 | |||||
| ATRA (50% w/w) | 74.0 | 28.3 | |||||
| rPAA-Chol | nanoparticle | siRNA | 9.7 (N/A) | N/A | N/A | 14.0 | [147,148] |
| 10.9 (N/A) | 29.0 | ||||||
| 13.5 (N/A) | 57.0 | ||||||
| 15.9 (N/A) | 87.0 | ||||||
| (PAE-g-Chol)-b-PEG-b-(PAE-g-Chol) | micelle | DOX (10% w/w) | N/A | 33.6 | 4.2 | 48.0 | [41] |
| DOX (20% w/w) | 48.7 | 13.5 | |||||
| DOX (50% w/w) | 59.5 | 20.1 | |||||
| DOX (80% w/w) | 55.3 | 24.3 | |||||
| PEG-PMMI-CholC6 | liposome | RAPA | 74.0 (1.51) | 76.9 | N/A | 4.9 | [42] |
| PEG-PMMI-CholC6 | liposome | MTX | N/A | 63.1 | N/A | N/A | [43] |
| PMMI-CholC6 | micelle | PX | 57.1 (1.60) | 30.0 | 6.2 | 4.9 | [44] |
| PEG-PMMI-CholC6 | 74.3 (1.51) | 40.3 | 8.3 | 16.4 | |||
| Chol-PEG22- hbPG35 | liposome | Atto 488 tetrazine | N/A | >40 | N/A | N/A | [45] |
| Alexa Fluor 594 azide | >40 | ||||||
| HA-Chol | micelle | α -TOC | N/A | 77.6 | 16.1 | 4.6 | [15] |
| CUR | 82.8 | 3.3 | |||||
| CoQ10 | 86.2 | 10.7 | |||||
| L-PGA-g-Chol | nanoparticle | HSA | N/A | N/A | N/A | 0.065 | [77] |
| PEI-CyD-g-Chol | micelle | DOX | N/A | N/A | 5.4 | 5.2 | [149] |
| 7.4 | 7.9 | ||||||
| 12.8 | 18.6 | ||||||
| Chol-AL-AG | liposome | N/A | 27.0 (N/A) | N/A | N/A | N/A | [150] |
| mPEG-Dlabile-PAE-g-Chol | micelle | DOX | N/A | 53.5 | 11.2 | 55 | [46] |
| Chol-XG | nanogel | PTX | 20 000 (N/A) | N/A | N/A | N/A | [151] |
| Dex-Chol | micelle | RAPA 10% | 43.8 (N/A) | 79.9 | 7.3 | 4 | [152] |
| RAPA 20% | 90.1 | 12.6 | |||||
| mPEG-b-P(MBC78-{g-DMDPTA36; g-Chol30}-co-LA110) | polyplex | miRNA-34a | 43.5 (N/A) | N/A | N/A | N/A | [47] |
| mPEG-b-P(MBC65-{g-DMDPTA11; g-Chol19; g-Morph6;}-co-LA120) | 35.4 (N/A) | ||||||
| Chol-PN | nanoparticle | MTX | N/A | N/A | 5.2 | 3.6 | [48] |
| 6.7 | 5.7 | ||||||
| 8.6 | 6.7 | ||||||
| mPEG-PLL-g-DHPA/Chol-PBA (DHPA:Chol-PBA 1:1) | 55.6 | 13.9 | |||||
| Dex-Chol | micelle | DOX | N/A | 41.5 | 6.3 | 13.0 | [153] |
| HIS-Dex-Chol (24% HIS graft ratio) | 46.1 | 7.6 | |||||
| HIS-Dex-Chol (46% HIS graft ratio) | 56.3 | 12.3 | |||||
| Chol-g-P(HEMA10-co-DEAEMA25)-b-PPEGMA10 | micelle | DOX (12.5% w/w) | 14.3 (1.47) | 20.0 | 4.1 | N/A | [49] |
| DOX (25% w/w) | 38.0 | 8.7 | |||||
| DOX (50% w/w) | 30.0 | 13.1 | |||||
| Chol-g-P(HEMA10-co-DEAEMA35)-b-PPEGMA10 | DOX (12.5% w/w) | 16.4 (1.54) | 25.0 | 4.5 | |||
| DOX (25% w/w) | 48.5 | 10.8 | |||||
| DOX (50% w/w) | 36.7 | 15.5 | |||||
| PAE-g-mPEG-Chol | micelle | DOX (25% w/w) | 8.8 (1.90) | 25.5 | 9.5 | 62.0 | [50] |
| DOX (50% w/w) | 60.0 | 28.3 | |||||
| DOX (100% w/w) | 52.8 | 30.7 | |||||
| HMW-Chol-g-AlgA | nanoparticle | acetamiprid | 112.2 (N/A) | 90.8 | N/A | 4.6 | [154] |
| MMW-Chol-g-AlgA | 64.5 (N/A) | 86.8 | 5.4 | ||||
| LMW-Chol-g-AlgA | 47.5 (N/A) | 81.0 | 5.7 | ||||
| P(NIPAAm-co-NHMAAm)-g-Chol | micelle | Py | 8.1 (1.40) | N/A | 0.4 | N/A | [155] |
| HPC-PEG-Chol-biotin | micelle | PTX | N/A | N/A | 8.4 | 3.6 | [51] |
| CNC-Chol | nanocrystal | FA | N/A | 58 | N/A | 17 | [156] |
| Chol-Imi-OS | nanoparticle | CUR | N/A | 17.8 | 4.2 | N/A | [157] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misiak, P.; Markiewicz, K.H.; Szymczuk, D.; Wilczewska, A.Z. Polymeric Drug Delivery Systems Bearing Cholesterol Moieties: A Review. Polymers 2020, 12, 2620. https://doi.org/10.3390/polym12112620
Misiak P, Markiewicz KH, Szymczuk D, Wilczewska AZ. Polymeric Drug Delivery Systems Bearing Cholesterol Moieties: A Review. Polymers. 2020; 12(11):2620. https://doi.org/10.3390/polym12112620
Chicago/Turabian StyleMisiak, Paweł, Karolina H. Markiewicz, Dawid Szymczuk, and Agnieszka Z. Wilczewska. 2020. "Polymeric Drug Delivery Systems Bearing Cholesterol Moieties: A Review" Polymers 12, no. 11: 2620. https://doi.org/10.3390/polym12112620
APA StyleMisiak, P., Markiewicz, K. H., Szymczuk, D., & Wilczewska, A. Z. (2020). Polymeric Drug Delivery Systems Bearing Cholesterol Moieties: A Review. Polymers, 12(11), 2620. https://doi.org/10.3390/polym12112620