Bio-Degradable Polyurethane Foams Produced by Liquefied Polyol from Wheat Straw Biomass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Liquefaction of wheat straw
2.3. Preparation of Polyurethane Foams
2.4. Characterization of Products
2.4.1. Liquefied Product
2.4.2. LWS-Formulated PU Foams
3. Results and Discussion
3.1. Chemical Composition of Wheat Straw
3.2. Wheat Straw Liquefaction and Product Properties
3.3. LWS-PU Foam Properties
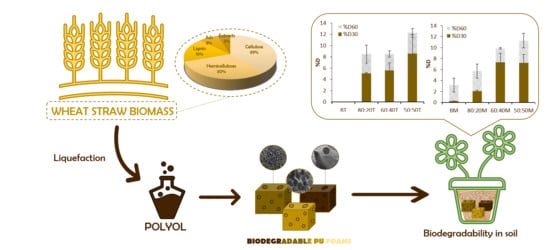
3.4. LWS-PU Foam Application: Biodegradability in Soil Media
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lozano, F.J.; Lozano, R. Assessing the potential sustainability benefits of agricultural residues: Biomass conversion to syngas for energy generation or to chemicals production. J. Clean. Prod. 2018, 172, 4162–4169. [Google Scholar] [CrossRef]
- Food and Agriculture Organization Corporate Statistical Database (FAOSTAT). New Food Balances. Available online: http://www.fao.org/faostat/en/#data/FBS (accessed on 2 December 2019).
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech. 2015, 5, 337–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa, E.; Tarrés, Q.; Delgado-Aguilar, M.; González, I.; Mutjé, P.; Rodríguez, A.; Espinosa, E.; Rodríguez, A. Suitability of wheat straw semichemical pulp for the fabrication of lignocellulosic nanofibres and their application to papermaking slurries. Cellulose 2015, 23, 837–852. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; Fermoso, J.; Herrador, E.; Hernando, H.; Jiménez-Sánchez, S.; Ballesteros, M.; González-Fernández, C.; Serrano, D. Valorization of steam-exploded wheat straw through a biorefinery approach: Bioethanol and bio-oil co-production. Fuel 2017, 199, 403–412. [Google Scholar] [CrossRef]
- Yang, S.; Bai, S.; Wang, Q. Sustainable packaging biocomposites from polylactic acid and wheat straw: Enhanced physical performance by solid state shear milling process. Compos. Sci. Technol. 2018, 158, 34–42. [Google Scholar] [CrossRef]
- Espinosa, E.; Bascón-Villegas, I.; Rosal, A.; Pérez-Rodríguez, F.; Chinga-Carrasco, G.; Rodríguez, A. PVA/(ligno)nanocellulose biocomposite films. Effect of residual lignin content on structural, mechanical, barrier and antioxidant properties. Int. J. Biol. Macromol. 2019, 141, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Sánchez, R.; Díaz-Carrasco, P.; Espinosa, E.; García-Domínguez, M.; Rodríguez, A. Isolation and characterization of lignins from wheat straw: Application as binder in lithium batteries. Int. J. Biol. Macromol. 2017, 104, 909–918. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Cerqueira, M.A.; Silva, H.D.; Rodríguez-Jasso, R.M.; Vicente, A.A.; Teixeira, J.A. Biorefinery valorization of autohydrolysis wheat straw hemicellulose to be applied in a polymer-blend film. Carbohydr. Polym. 2013, 92, 2154–2162. [Google Scholar] [CrossRef] [Green Version]
- Behrendt, F.; Neubauer, Y.; Oevermann, M.; Wilmes, B.; Zobel, N. Direct Liquefaction of Biomass. Chem. Eng. Technol. 2008, 31, 667–677. [Google Scholar] [CrossRef]
- Jiang, W.; Kumar, A.; Adamopoulos, S. Liquefaction of lignocellulosic materials and its applications in wood adhesives—A review. Ind. Crop. Prod. 2018, 124, 325–342. [Google Scholar] [CrossRef]
- Gómez-Jiménez-Aberasturi, O.; Ochoa-Gómez, J.R. New approaches to producing polyols from biomass. J. Chem. Technol. Biotechnol. 2017, 92, 705–711. [Google Scholar] [CrossRef]
- Tanaka, R.; Hirose, S.; Hatakeyama, H. Preparation and characterization of polyurethane foams using a palm oil-based polyol. Bioresour. Technol. 2008, 99, 3810–3816. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, J.; Cinelli, P.; Anguillesi, I.; Coltelli, M.-B.; Lazzeri, A. Flexible polyurethane foams green production employing lignin or oxypropylated lignin. Eur. Polym. J. 2015, 64, 147–156. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Hu, L.; Zhou, Y. Synthesis of rigid polyurethane foams with castor oil-based flame retardant polyols. Ind. Crop. Prod. 2014, 52, 380–388. [Google Scholar] [CrossRef]
- Trevino, A.; Trumbo, D. Acetoacetylated castor oil in coatings applications. Prog. Org. Coatings 2002, 44, 49–54. [Google Scholar] [CrossRef]
- Technical Association of the Pulp and Paper Industry (TAPPI). TAPPI Standards: Regulation and Style Guidelines. Revised January 2018. Available online: http://www.tappi.org/content/pdf/standards/tm_guidelines_complete.pdf (accessed on 10 November 2020).
- Briones, R.; Serrano, L.; Labidi, J. Valorization of some lignocellulosic agro-industrial residues to obtain biopolyols. J. Chem. Technol. Biotechnol. 2011, 87, 244–249. [Google Scholar] [CrossRef]
- Briones, R.; Serrano, L.; Llano-Ponte, R.; Labidi, J. Polyols obtained from solvolysis liquefaction of biodiesel production solid residues. Chem. Eng. J. 2011, 175, 169–175. [Google Scholar] [CrossRef]
- Li, H.; Feng, S.; Yuan, Z.; Wei, Q.; Souzanchi, S. Highly efficient liquefaction of wheat straw for the production of bio-polyols and bio-based polyurethane foams. Ind. Crop. Prod. 2017, 109, 426–433. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kwon, O.-J.; Yang, S.-R.; Park, J.; Chun, B.C. Structural, thermal, and mechanical properties of polyurethane foams prepared with starch as the main component of polyols. Fibers Polym. 2007, 8, 155–162. [Google Scholar] [CrossRef]
- Rincón, E.; Balu, A.M.; Luque, R.; Serrano, L. Insulating rigid polyurethane foams from laurel tree pruning based polyol. J. Appl. Polym. Sci. 2020, 138, 49789. [Google Scholar] [CrossRef]
- ASTM D4274-16, Standard Test Method for Testing Polyurethane Raw Materials: Determination of Hydroxyl Numbers of Polyols; ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D1622/D1622M-14, Standard Method For Apparent Density of Rigid Cellular Plastics; ASTM International: West Conshohocken, PA, USA, 2008.
- ASTM D1621-16, Standard Test Method for Compressive Properties of rigid Cellular Plastics; ASTM International: West Conshohocken, PA, USA, 2016.
- Huang, G.; Wang, P. Effects of preparation conditions on properties of rigid polyurethane foam composites based on liquefied bagasse and jute fibre. Polym. Test. 2017, 60, 266–273. [Google Scholar] [CrossRef]
- ASTM D5988-03, Standard Test Method for Determining Aerobic Biodegradation in Soil of Plastic Materials or Residual Plastic Materials After Composting; ASTM International: West Conshohocken, PA, USA, 2003.
- Kurańska, M.; Prociak, A. The influence of rapeseed oil-based polyols on the foaming process of rigid polyurethane foams. Ind. Crop. Prod. 2016, 89, 182–187. [Google Scholar] [CrossRef]
- Zlatanić, A.; Lava, C.; Zhang, W.; Petrović, Z.S. Effect of structure on properties of polyols and polyurethanes based on different vegetable oils. J. Polym. Sci. Part. B Polym. Phys. 2004, 42, 809–819. [Google Scholar] [CrossRef]
- Zhang, J.; Hori, N.; Takemura, A. Influence of NCO/OH ratio on preparation of four agricultural wastes liquefied polyols based polyurethane foams. Polym. Degrad. Stab. 2020, 179, 109256. [Google Scholar] [CrossRef]
- Kirpluks, M.; Kalnbunde, D.; Benes, H.; Cabulis, U. Natural oil based highly functional polyols as feedstock for rigid polyurethane foam thermal insulation. Ind. Crop. Prod. 2018, 122, 627–636. [Google Scholar] [CrossRef]
- Cinelli, P.; Anguillesi, I.; Lazzeri, A. Green synthesis of flexible polyurethane foams from liquefied lignin. Eur. Polym. J. 2013, 49, 1174–1184. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, Y.; Xie, Y.; Qiao, K.; Sun, Y.; Yue, L. Synthesis of bio-castor oil polyurethane flexible foams and the influence of biotic component on their performance. J. Polym. Res. 2015, 22, 1–9. [Google Scholar] [CrossRef]
- Tu, Y.-C.; Suppes, G.J.; Hsieh, F.-H. Water-blown rigid and flexible polyurethane foams containing epoxidized soybean oil triglycerides. J. Appl. Polym. Sci. 2008, 109, 537–544. [Google Scholar] [CrossRef]
- Mosiewicki, M.; Dell’Arciprete, G.; Aranguren, M.; Marcovich, N. Polyurethane Foams Obtained from Castor Oil-based Polyol and Filled with Wood Flour. J. Compos. Mater. 2009, 43, 3057–3072. [Google Scholar] [CrossRef]
- Hejna, A.; Kirpluks, M.; Kosmela, P.; Cabulis, U.; Haponiuk, J.; Łukasz, P. The influence of crude glycerol and castor oil-based polyol on the structure and performance of rigid polyurethane-polyisocyanurate foams. Ind. Crop. Prod. 2017, 95, 113–125. [Google Scholar] [CrossRef]
- Wang, H.J.; Rong, M.Z.; Zhang, M.Q.; Hu, J.; Chen, H.W.; Czigány, T. Biodegradable Foam Plastics Based on Castor Oil. Biomacromolecules 2008, 9, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, H.; Hojati-Talemi, P. Preparation and properties of novel biodegradable polyurethane networks based on castor oil and poly(ethylene glycol). Polym. Degrad. Stab. 2007, 92, 480–489. [Google Scholar] [CrossRef]







| Sample Name | Formulation (CO: LWS) | CO (g) | LWS (g) | TDI (g) | MDI (g) | RNCO/OH | Cream Time (s) | Free Rise Time (s) |
|---|---|---|---|---|---|---|---|---|
| BT | Blank | 20 | 0 | 11.5 | 0 | 0.554 | 42 | 72 |
| 80:20T | 80:20 | 16 | 4 | 11.5 | 0 | 0.647 | 50 | 98 |
| 60:40T | 60:40 | 12 | 8 | 11.5 | 0 | 0.779 | 111 | 173 |
| 50:50T | 50:50 | 10 | 10 | 11.5 | 0 | 0.867 | 102 | 148 |
| BM | Blank | 20 | 0 | 0 | 11.5 | 0.386 | 5.36 | 14.87 |
| 80:20M | 80:20 | 16 | 4 | 0 | 11.5 | 0.451 | 7.43 | 25.96 |
| 60:40M | 60:40 | 12 | 8 | 0 | 11.5 | 0.542 | 24.94 | 59.81 |
| 50:50M | 50:50 | 10 | 10 | 0 | 11.5 | 0.603 | 51 | 72 |
| Sample | Yield (%) | pH (25 °C) | IOH (mg KOH/g) | Acid number (mg KOH/g) | Viscosity (Pa·s) | Mw (g/mol) | Mn (g/mol) |
|---|---|---|---|---|---|---|---|
| LWS | 96.5 | 1.63 ± 0.02 | 604.1 ± 9.1 | 59.2 ± 0.86 | 0.6 ± 0.05 | 30,463 | 28,170 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, L.; Rincón, E.; García, A.; Rodríguez, J.; Briones, R. Bio-Degradable Polyurethane Foams Produced by Liquefied Polyol from Wheat Straw Biomass. Polymers 2020, 12, 2646. https://doi.org/10.3390/polym12112646
Serrano L, Rincón E, García A, Rodríguez J, Briones R. Bio-Degradable Polyurethane Foams Produced by Liquefied Polyol from Wheat Straw Biomass. Polymers. 2020; 12(11):2646. https://doi.org/10.3390/polym12112646
Chicago/Turabian StyleSerrano, Luis, Esther Rincón, Araceli García, Jesús Rodríguez, and Rodrigo Briones. 2020. "Bio-Degradable Polyurethane Foams Produced by Liquefied Polyol from Wheat Straw Biomass" Polymers 12, no. 11: 2646. https://doi.org/10.3390/polym12112646
APA StyleSerrano, L., Rincón, E., García, A., Rodríguez, J., & Briones, R. (2020). Bio-Degradable Polyurethane Foams Produced by Liquefied Polyol from Wheat Straw Biomass. Polymers, 12(11), 2646. https://doi.org/10.3390/polym12112646