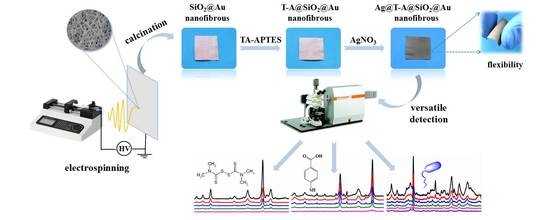
3.1. Preparation and Characterization of Ag@T-A@SiO2@Au Nanofibers
Figure 2 shows SEM images for nanofibers of SiO
2@Au-10 precursor (a), SiO
2@Au-20 precursor (b), SiO
2@Au-30 precursor (c), SiO
2@Au-10 (d), SiO
2@Au-20 (e), SiO
2@Au-30 (f), T-A@SiO
2@Au-20 (g), Ag@T-A@SiO
2@Au-20 (h); and EDS spectra of Ag@T-A@SiO
2@Au-20 nanofibers (i). It can be seen from
Figure 2a–c that the surface of SiO
2@Au precursor nanofibers with different Au content is smooth and flat, and the fibers are interlaced in a horizontal and vertical manner to form a three-dimensional network structure. In contrast, the SiO
2@Au nanofibers keep the smooth, even, and uniform surface morphology of precursor nanofibers after calcination, as shown in
Figure 2d–f. It is worth noting that in
Figure 2d,e, no particles are observed on the surface of the nanofibers. However, it can be clearly seen from
Figure 2f that nanoparticles emerged on the surface of SiO
2@Au-30 nanofibers, accompanied by the increase in Au content. This is because the excitation electrons have a limited ability to penetrate SiO
2 nanofibers under an acceleration voltage of 5 kV, such that Au nanoparticles embedded in the fibers cannot be imaged. Nevertheless, if too much chloroauric acid is added, some Au nanoparticles will be released from the inside of the fibers after electrospinning and calcination. Since the purpose of this study is to prepare a bimetallic nanoparticle Raman enhanced substrate, Au nanoparticles are required to be embedded in SiO
2 nanofibers and have a high content. Therefore, it is more appropriate to select SiO
2@Au-20 samples for the further work. Compared with
Figure 2a–c and
Figure 2d–f, it is found that the average diameters of nanofibers are significantly decreased after calcination. Taking the sample of SiO
2@Au-20 as an example, it can be seen that the mean diameters of nanofibers before and after calcination are decreased from 358 ± 30 to 218 ± 29 nm, as shown in
Figure S1a,b. The decrease in fibers’ diameter is due to the removal of a large amount of organic matter (such as PVP), dehydration of SiO
2 precursor, and the decomposition of HAuCl
4 during the calcination, leaving only Au nanoparticles embedded in SiO
2 nanofibers, which can be furtherly proved in the XRD analysis. From
Figure 2g, it can be seen that after the TA-APTES modification, some nanofibers are bent in shape and the surfaces of nanofibers are not ever smooth but change to a rough morphology. The formation of this rough coating on the surfaces of SiO
2@Au nanofibers can be attributed to the Michael addition reaction between the oxidation product of TA and the hydrolysis product of APTES [
25]. Correspondingly, it can be seen from
Figure S1c that the diameter of T-A@SiO
2@Au-20 nanofibers increased to 267 ± 31 nm. Moreover, it is seen from
Figure 2h that after 30 min of immersion in 0.1 mol/L AgNO
3 solution, a large number of nanoparticles formed and were deposited on the surfaces of T-A@SiO
2@Au-20 fibers, which causes the nanofibers’ diameter to increase to 355 ± 33 nm, as shown in
Figure S1d. Obviously, Ag nanoparticles are coated on the surface of nanofibers. It is supposed that the formation process is as follows: the modification layer of TA-APTES contains a large number of hydroxyl and amino groups, which can chelate Ag
+, and the hydroxyl group in TA can reduce Ag
+ to Ag nanoparticles in situ. It also can be found from
Figure 2h that these Ag nanoparticles cover almost the entire surface of the fibers uniformly and densely, and the gaps between adjacent Ag nanoparticles are in the nanometer scale, which facilitates the formation of SERS hot spots, and improves the SERS sensitivity. As a micro-domain element analysis tool, EDS spectroscopy can be applied to confirm the elemental composition of the composite nanofibers.
Figure 2i verifies the existence of Si, O, C, N, Au, and Ag elements in Ag@T-A@SiO
2@Au-20 nanofibers. It is worth pointing out that the percentage of Ag atoms is rather high, which helps to improve its SERS effect.
In order to more clearly reveal the microstructure of the composite nanofibers and confirm the incorporation of Au nanoparticles inside the fibers, the Ag@T-A@SiO
2@Au nanofibers were characterized by TEM.
Figure 3 shows TEM images of SiO
2@Au-10 (a), SiO
2@Au-20 (b), SiO
2@Au-30 (c), T-A@SiO
2@Au-20 (d), Ag@T-A@SiO
2@Au-20 (e) nanofibers, and the local enlarged cross-sectional view of Ag@T-A@SiO
2@Au-20 (e). As can be seen from
Figure 3a, when the added amount of HAuCl
4 is 10 mg, the surface of SiO
2@Au-10 nanofibers is smooth and flat, and the distribution of Au nanoparticles inside SiO
2 is relatively scattered, while the average particle size of Au nanoparticles is about 6.1 nm, as shown in
Figure S2a. When the added amount of HAuCl
4 is increased to 20 mg, it can be seen from
Figure 3b that a large number of Au nanoparticles are evenly distributed inside SiO
2 nanofibers, and these Au nanoparticles are spherical with an average particle size of 8.2 nm, as shown in
Figure S2b. However, it can be seen from
Figure 3c that Au nanoparticles are formed inside and outside of SiO
2 nanofibers when the added amount of HAuCl
4 is further increased to 30 mg, and the agglomeration of Au nanoparticles is obviously observed. Correspondingly, the size of the Au nanoparticles increased to 15.7 nm, as shown in
Figure S2c. Therefore, in this study, the SiO
2@Au-20 nanofibers with a large load density and uniform distribution of Au nanoparticles were selected for the next step of sample preparation. It can be seen from
Figure 3d that after TA-APTES modification, a thin layer of rough coating appears on the surface of the nanofibers and some aggregates also occurred, which is consistent with the SEM results. It can be found from
Figure 3e that Ag nanoparticles are formed on the surface of T-A@SiO
2@Au-20 nanofibers after it is immersed in AgNO
3 solution, and the mean particle size of Ag nanoparticles is about 33.2 nm, as shown in
Figure S2d. Moreover, the dense and uniform distribution of Au nanoparticles inside the nanofibers and the successful decoration of Ag nanoparticles on the fiber surface can be clearly distinguished from the local enlarged cross-section view, as shown in
Figure 3f.
In order to further confirm that the surface of nanofibers was successfully modified, AFM was used to characterize the surface roughness changes of SiO
2 composite nanofibrous membranes before and after modification.
Figure S3 shows the three-dimensional and two-dimensional AFM images of SiO
2@Au-20 and Ag@T-A@SiO
2@Au-20 electrospun nanofibrous membranes. It can be seen from
Figure S3a,c that the surface of the SiO
2@Au-20 nanofibers is smooth and flat, and these nanofibers randomly crisscross to form a three-dimensional network structure. However, after the surface modification, it can be clearly seen from
Figure S3b,d that the fiber surface is no longer smooth, but coarse particles appear. In addition, it can be seen from
Figure S3 that the arithmetic mean deviation of the profile (
Ra) changed from 185 before modification to 220 after treatment, indicating that the surface roughness of the nanofibrous membrane increased significantly.
Based on the above SEM, TEM, and AFM observation, the morphology and structure of Au and Ag bimetallic nanoparticles decorated inside and outside of SiO2 nanofibers is clearly revealed. It is supposed that the as-prepared Ag@T-A@SiO2@Au composite nanofibers would exhibit excellent SERS activities. This is due to the fact that the internal Au nanoparticles possess high stability during SERS detection, and external Ag nanoparticles have a large number of SERS “hot spots”, and the bimetallic nanoparticles will form a synergistic enhancement effect.
The crystal structure of Au and Ag nanoparticles was determined by XRD.
Figure 4a shows the diffraction patterns of SiO
2@Au-20, T-A@SiO
2@Au-20 and Ag@T-A@SiO
2@Au-20 nanofibers, respectively. As seen from
Figure 4a, SiO
2@Au-20 nanofibers exhibit diffraction peaks at 23.1°, 38.1°, 44.3°, 64.3°, 77.4°, and 81.5°. Among of them, the diffraction peak at 23.1° belongs to the amorphous SiO
2 phase, while the peaks at 2θ = 38.1°, 44.3°, 64.3°, 77.4°, and 81.5°are assigned to the (111), (200), (220), (311) and (222) crystal planes of face-centered cubic (fcc) Au (JCPDS, No. 04-0784), respectively [
7,
23]. After the TA-APTES modification, the diffraction peaks of T-A@SiO
2@Au-20 nanofibers do not show significant changes, indicating that the modification TA-APTES on the nanofiber’s surfaces has no effect on the crystal structure of SiO
2@Au nanofibers. However, when Ag nanoparticles were further decorated on the nanofiber’s surface, the XRD spectrum of the Ag@T-A@SiO
2@Au-20 nanofibers show similar diffraction patterns consistent with the SiO
2@Au-20 nanofibers, except for the obvious enhancement of the peak’s intensity. This is because the diffraction peak positions of fcc Ag (JCPDS, No. 04-0783) [
26] are almost as same as those of fcc Au. Meanwhile, the dense deposition of Ag nanoparticles on the surface of nanofibers increases the intensity of diffraction peaks.
The thermal decomposition process of nanofibrous membranes were detected by the technique of TGA.
Figure 4b shows TGA curves of SiO
2@Au-20 precursor, SiO
2@Au-20, T-A@SiO
2@Au-20 and Ag@T-A@SiO
2@Au-20 nanofibers. For the sample of SiO
2@Au-20 precursor nanofibers, there are three stages of decomposition. The first step occurs from 25 to 100 °C, and the weight loss is about 7.1%. This is due to the volatilization of adsorbed water and the residual solvent in the nanofiber’s precursor. The second step occurs from 100 to 350 °C; the sample loses 7.7% of its mass, which can be attributed to the dehydration of TEOS, the decomposition of PVP side chains and HAuCl
4. The last step with a significant mass loss occurs from 350 to 500 °C—the weight loss is about 50.1%, which is assigned to the decomposition of the PVP skeleton and the condensation reaction of TEOS [
23,
27]. Note that there is almost no mass loss for the SiO
2@Au precursor nanofibers after 500 °C, suggesting the complete formation of SiO
2@Au nanofibers. Based on the result obtained above, a high-temperature calcination of 600 °C was selected to obtain SiO
2@Au nanofibers from the as-prepared electrospun SiO
2@Au-20 precursor nanofibers in this study. Comparing the TGA curves of SiO
2@Au-20 and T-A@SiO
2@Au-20 nanofibers, it can be found that the total mass loss of SiO
2@Au-20 and T-A@SiO
2@Au-20 nanofibers is 15.8% and 23.5%, respectively. The mass loss difference value is about 7.7%, which is obviously due to the thermo decomposition of the TA-APTES modification layer. In addition, it can be seen from the TGA curve of Ag@T-A@SiO
2@Au-20 nanofibers that the Ag nanoparticles decorated on the surface of the nanofibers do not decompose, and the total weight loss is about 11.5%, even when the temperature is raised to 800 °C. Generally, the as-prepared Ag@T-A@SiO
2@Au nanofiber membranes in this work exhibit high thermal stability. Good thermal stability is one of the key advantages of SERS substrates, since they can endure laser radiation.
In addition, the XPS analysis method was applied to further explore the surface chemical structure of electrospun nanofibrous membranes for verification of the modification of TA-APTES and Ag nanoparticles.
Figure 5 shows the XPS full spectra (a) of SiO
2@Au-20 and Ag@T-A@SiO
2@Au-20 nanofibers, and the divided peak spectra of Au (b), Ag (c), C (d), N (e). Comparing the two XPS full spectra in
Figure 5a, it can be clearly observed that after modification with TA-APTES and Ag nanoparticles, apart from Si, O, and Au, there are new signal peaks of C, N, and Ag in Ag@T-A@SiO
2@Au-20 nanofibers, which is consistent with the chemical composition of the composite nanofibers. It is worth noting that the signal peak of Au is very weak in the spectrum of nanofibers. The reason may be the relatively low content of Au atoms in the nanofibers, as shown in
Figure 2i above. In addition, XPS is a surface analysis tool, and Au nanoparticles are incorporated into the fiber, which affects its peak intensity to a certain extent. From the high-resolution XPS spectrum of the Au 4f orbital region (
Figure 5b), it can be seen that the binding energy peaks of Au 4f
7/2 and Au 4f
5/2 are 83.4 and 87.1 eV, respectively. The splitting energy of the 4f doublet is 3.7 eV, which indicates the existence metallic state of Au
0 [
28]. Meanwhile, in the spectrum of Ag 3d, as shown in
Figure 5c, the typical peaks of Ag 3d
5/2 and Ag 3d
3/2 appear at 368.2 and 374.2 eV, with a spin-orbit separation of 6.0 eV, which can be attributed to the metallic state of Ag, implying that the metallic state of Ag formed on the surface of the SiO
2 nanofibers [
29].
Figure 5d shows the sub-peaks corresponding to C 1s. There are six peaks at 283.6, 284.1, 284.5, 285.1, 286.2 and 287.8 eV in the C 1s whole spectrum, which are attributed to C–Si, C = C, C−C, C−N, C−OH and C = O bonds, respectively [
30]. In the N 1s spectrum of
Figure 5e, the peaks of 399.5 and 401.7 eV correspond to the N−C, −NH− bond, respectively [
25,
31]. The appearance of C−N and −NH− bonds further confirms that the Michael addition reaction between TA and APTES does occur. This reaction can produce a rough surface coating similar to a binder on the fiber surface. The coating can play the role of bridging, and can firmly bond the SiO
2@Au substrate with the Ag nanoparticles, so that the substrate has high detection sensitivity and excellent stability during the SERS detection process. Combined with the above results of the SEM, TEM and XRD analysis, the XPS spectra confirm once again the chemical structure of Au/Ag bimetallic nanoparticles modified SiO
2 nanofibers.
Figure 6 shows the photographs of nanofibrous membranes SiO
2@Au-20 (a,d), T-A@SiO
2@Au-20 (b,e), Ag@T-A@SiO
2@Au-20 (c,f) before and after manual folding. From
Figure 6a–c, it can be clearly seen that after the modification by TA-APTES and Ag nanoparticles, the color of SiO
2@Au membranes changes from the original pink to light red, and then to black, indicating that TA-APTES and Ag nanoparticles are deposited on the surface of the nanofibrous membranes. Simultaneously, a flexibility experiment was performed on the as-prepared membranes by manual bending, as displayed in
Figure 6d–f. It can be clearly seen that the SiO
2@Au nanofibrous membranes can be bent 180° without breaking after being calcined at a high temperature of 600 °C. Furthermore, the samples modified by TA-APTES and Ag nanoparticles also maintain the good flexibility of SiO
2@Au nanofibers. This good flexibility ensures that the electrospun layered nanofibrous membrane is not easy to break, combined with the large specific surface area and porosity feature, meaning the substrates can collect trace amounts of target analyte molecules effectively, which is a critical factor which has been neglected in practical SERS application [
32,
33].
3.2. SERS Activities for Small Molecules
To evaluate the SERS activity, comparative experiments were conducted by recording SERS spectra of 4-MPh and 4-MBA adsorbed on SiO
2@Au, Ag@T-A@SiO
2, and Ag@T-A@SiO
2@Au nanofibers, as shown in
Figure 7.
Figure 7a presents the SERS spectra of 4-MPh (10
−1 mol/L) molecules collected on SiO
2@Au nanofibers with different HAuCl
4 contents. It can be seen from
Figure 7a that 4-MPh molecules adsorbed on the SiO
2@Au nanofibrous membranes can all produce obvious SERS peaks. The SERS peaks at 390, 638, 824, 1007, 1073, 1490 and 1596 cm
−1 correspond to the stretching and bending vibrations of the groups in the 4-MPh molecule [
34]. By the comparison of the spectra for different samples in
Figure 7a, it can be found that the 4-MPh SERS signals collected by the SiO
2@Au-20 sample are the strongest. The best SERS effect can be attributed to the uniform distribution and high load density of Au nanoparticles in the fibers, as revealed by the above TEM analysis. The probes molecules can diffuse into the fibers and in full contact with Au nanoparticles to produce more hot spots, which is conducive to the provision of SERS signals. In addition, in order to verify that the as-prepared nanofibrous membrane substrate itself has no characteristic Raman peaks that interfere with SERS detection, Raman analysis of neat films without analytes was conducted. It can be seen from
Figure 7a,b that there is no Raman signal from SiO
2@Au-20 membranes without analytes. This feature helps in the simplification of SERS detection. Furthermore, to investigate the SERS enhancement properties of the modified nanofibers, 4-MPh molecules at a concentration of 10
−5 mol/L, were detected by substrates of SiO
2@Au-20, Ag@T-A@SiO
2 and Ag@T-A@SiO
2@Au-20, as shown in
Figure 7c. Comparing the SERS spectra of three different samples in
Figure 7c, it is found that the 4-MPh SERS peak intensity of Ag@T-A@SiO
2@Au-20 composite nanofibrous membranes substrate is the highest, indicating that this sample has the most significant Raman enhancement effect on 4-MPh. The reason can be attributed to the fact that Au and Ag electromagnetic field synergistic enhancement in nanofibers can provide abundant SESR hot spots. The laser can not only irradiate Ag nanoparticles on the fibers’ surface, but also pass through the surface SiO
2 nanofibers and come into contact with the embedded Au nanoparticles, thus causing plasmon resonance [
10]. Similarly,
Figure 7b,d show the SERS detection results of the as-prepared samples on another probe molecule, i.e., 4-MBA. It can be perceived from
Figure 7b that the SERS peaks centered at 523, 1080, 1186, and 1587 cm
−1 are attributed to the characteristic Raman absorption of 4-MBA adsorbed on the fibrous samples [
35], and the SERS signal intensity of the SiO
2@Au-20 nanofibers is significantly higher than the other two samples. In addition, it can also be found that Ag@T-A@SiO
2@Au-20 nanofibers show the strongest SERS effect in three different samples, as shown in
Figure 7d, which is consistent with the results of 4-MPh. Combining the morphology and structure analysis with SERS results, it is demonstrated that Ag@T-A@SiO
2@Au-20 nanofibrous membranes possess the optimal SERS activities, and these membranes were selected for the detection of other analytes in the coming experiment.
In order to further investigate the SERS performance of Ag@T-A@SiO
2@Au-20 nanofibrous substrates, the Raman spectra of 4-MPh molecules with different concentrations (from 10
−3 to 10
−11 mol/L) on the substrate are detected, as shown in
Figure 8a. It is found that the peak intensities decrease with the decay of the 4-MPh concentration. However, a well-resolved Raman spectrum can still be clearly observed, even when the concentration is as low as 10
−11 mol/L. This means that Ag@T-A@SiO
2@Au-20 electrospun nanofibrous membranes as the SERS substrate have a very high detection sensitivity for 4-MPh. Meanwhile, in order to verify the applicability of the substrate to different probe molecules, similar detection was performed on 4-MBA molecules. For this molecule, except for the fact that the characteristic peak position changed, the other SERS detection results are similar to 4-MPh. It can be found from
Figure 8d that the peak intensity is related to the concentration of the probe molecules, and the detection limit for 4-MBA also reaches 10
−11 mol/L. Additionally, the SERS EF on the Ag@T-A@SiO
2@Au-20 nanofibrous membranes was caudated by using 4-MPh and 4-MBA as the target analytes. In this test, Ag@T-A@SiO
2 nanofibers that adsorbed 10
−11 mol/L probe molecules and the blank silicon wafer that adsorbed 10
−3 mol/L probe molecules are compared. The EFs of the nanofibrous platform are calculated as 5.4 × 10
8 for 4-MPh and 2.3 × 10
8 for 4-MBA, respectively (the detail data are shown in
Table S1). Compared with the different electrospun SERS substrates reported by other researchers, as shown in
Table S2, the as-prepared Ag@T-A@SiO
2@Au-20 nanofibrous substrates possess a higher EF. Obviously, this can be attributed to the synergistic Raman enhancement effect of the bimetallic nanoparticles. The above-mentioned results adequately prove that the as-prepared electrospun nanofibrous membranes have the capability to act as an excellent SERS substrate, and can realize trace detection for small probe molecules, which are higher than our previous reports [
16,
17] and other test results of similar Ag composite nanostructures [
36].
In order to further study the stability of SERS detection for the as-prepared Ag@T-A@SiO
2@Au-20 nanofibrous membranes, a durability test was performed through consecutively washing the substrates by absolute ethanol after the analytes were adsorbed.
Figure 8b,e show the SERS spectra for 4-MPh and 4-MBA (10
−5 mol/L) versus cleaning times. Correspondingly, the intensity of the strongest peak in 4-MPh and 4-MBA (i.e., 1073 and 1587 cm
−1) changes with washing time, as shown in
Figure 8c,f, respectively. It is found that the characteristic Raman peak intensity decreases slowly with the increase in washing time. However, even after five consecutive washings with ethanol, the clearly visible characteristic Raman peak can still be detected. This proves that Ag@T-A@SiO
2@Au-20 nanofibrous membranes provide high SERS detection stability, which is due to the incorporation of Au nanoparticles into SiO
2 nanofibers and firm binding of Ag nanoparticles through chemical bonding by TA-APTES.
Rapid detection and identification of toxic substances in water or an aquatic environment is one of the important tasks in SERS analysis [
37]. After verifying the high SERS detection sensitivity through small probe molecules, for practical applications, the as-prepared nanofibrous membranes were also used as SERS substrates for pesticide detection.
Figure 9a shows the SERS spectra of thiram with concentrations increasing from 10
−8 to 10
−3 mol/L. From
Figure 9a, it can be found that the Raman characteristic peaks of thiram appear at 560, 928, 1150, 1386 and 1514 cm
−1, respectively, which are consistent with the results reported in the literature [
38]. At the same time, within the concentration range shown in this figure, the intensity of characteristic peak at 1386 cm
−1 was used as the quantitative basis to evaluate the SERS sensitivity. The results show that thiram can still be clearly identified at a low concentration of 10
−8 mol/L, showing better sensitivity than other Ag nanostructured SERS substrates previously reported [
1]. It is worth mentioning that this detection limit is lower than the U.S. Environmental Protection Agency’s standard requirements for the allowable minimum residue concentration of pesticides thiram [
39], so it can be used for trace detection of this pesticide. For the actual application, the stability of SERS substrate is an important issue to be considered in use. The stability of the as-prepared substrate was further investigated by durability test. The nanofibrous SERS substrates prepared in the same batch were soaked in 10
−5 mol/L thiram solution, dried and stored in air for 60 days, and SERS detection was performed every 10 days, and the results are shown in
Figure 9b,c. It can be seen from
Figure 9b that the peaks positions and intensities are the same. In addition, the characteristic peak intensity of thiram at 1386 cm
−1 preserved for 60 days was analyzed. It was found that the SERS signal intensity decreased by only 14.1%, when the storage time was 60 days. This result is superior to that of the similar work [
29,
40]. This good detection sensitivity and stability can be attributed to the following facts. On the one hand, it is related to the molecule structure of thiram, since the S–S bond of thiram can be cleaved into two methylene residues, which can be strongly adsorbed into the three-dimensional network electrospun nanofibrous membranes [
41]. On the other hand, it is well known that inner Au nanoparticles have good stability, and the external Ag nanoparticles are bonded with hydroxyl and amino groups of TA-APTES, which can prevent oxidation of metal nanoparticles. Therefore, the stable Au and Ag nanoparticles can keep their surface plasma resonance activities for a long time, so that the SERS substrates have good SERS detection stability.
3.3. SERS Performance for Bacteria Detection
SERS detection of small molecule probes and pesticide thiram demonstrates that the as-prepared nanofibrous substrates have excellent detection sensitivity and good stability. Next,
S. aureus was selected as the target strain to verify the feasibility of substrate detection of biomacromolecules. The SERS peaks of bacteria are mainly derived from proteins, polysaccharides, nucleic acids, carbohydrates and lipids in bacterial cell structure [
42,
43]. Therefore, through SERS spectrum matching, different bacterial structure information can be obtained to distinguish bacteria. Before SERS detection, the adsorption of bacterial strains on the electrospun nanofibrous membrane was first observed by SEM.
Figure S4 shows SEM images for different magnifications of
S aureus attached on the Ag@T-A@SiO
2@Au-20 nanofibrous membranes. It can be seen from
Figure S4 that a large number of
S. aureus strains are adsorbed on the fibrous membranes. The reason is that the electrospun nanofibrous membranes have a relatively large specific surface area and porosity, which is beneficial to physical adsorption, and the surface modification layer of TA-APTES contains a large number of hydroxyl and amino groups that can bind to functional groups on the surface of bacterial cells. In order to verify the repeatability of the SERS substrates, 20 points were randomly selected on the Ag@T-A@SiO
2@Au-20 nanofibrous membranes, and the SERS spectra of
S. aureus (10
9 cfu/mL) were measured under the same conditions, as shown in
Figure 10a. It can be found from this figure that all SERS spectra show a high degree of uniformity in both the position and intensity of peaks. There are several main SERS peaks at 733, 1327, 1444 and 1576 cm
−1, corresponding to the vibrational absorption of adenine, guanine, saturated lipids and amides in proteins [
23]. At the same time, as shown in
Figure 10b, the peak intensity at 733 cm
−1 remains stable, and the relative standard deviation (RSD) is calculated to be 6.1%, indicating the good homogeneity or repeatability of the as-prepared Ag@T-A@SiO
2@Au-20 substrates.
So far, it has been confirmed that the as-prepared electrospun nanofibrous membrane SERS substrate has excellent sensitivity, good stability, and superior repeatability. Furthermore, the relationship between the SERS peak intensity and the concentration of the bacterial suspension was established in order to realize the quantitative analysis of bacteria.
Figure 10c shows the SERS spectra of
S. aureus with different concentrations (from 10
3 to 10
8 cfu/mL). As seen from
Figure 10c, the spectra of different concentrations of
S. aureus adsorbed on the Ag@T-A@SiO
2@Au-20 nanofibrous membranes show significant enhanced Raman signals. Taking the characteristic Raman peak at 733 cm
−1 as an example, it can be seen that the peak intensity decreased gradually with the decrease in bacterial concentration. However, this SERS peak can be clearly distinguished even if the concentration is as low as 10
3 cfu/mL. More importantly,
Figure 10d shows the relationship plot between the peak intensity and the logarithm of
S. aureus concentration. It can be seen from
Figure 10d that there is a good linear correlation between the peak intensity at 733 cm
−1 and the logarithm of bacterial concentration, and the correlation coefficient is calculated to be 0.9461. Compared with the related literature [
44], the result of this work is superior. Based on the above SERS analysis results for bacteria, it is demonstrated that the as-prepared Ag@T-A@SiO
2@Au-20 nanofibrous membrane substrate can directly obtain the characteristic Raman spectra of bacteria without a complicated ligand binding process, and possess ultra-high detection sensitivity and excellent uniformity. It is worth emphasizing that in addition to qualitative identification, this substrate can also perform quantitative SERS detection of biological macromolecules, making it more practical for application.