Polyurethane-Based Composites: Effects of Antibacterial Fillers on the Physical-Mechanical Behavior of Thermoplastic Polyurethanes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
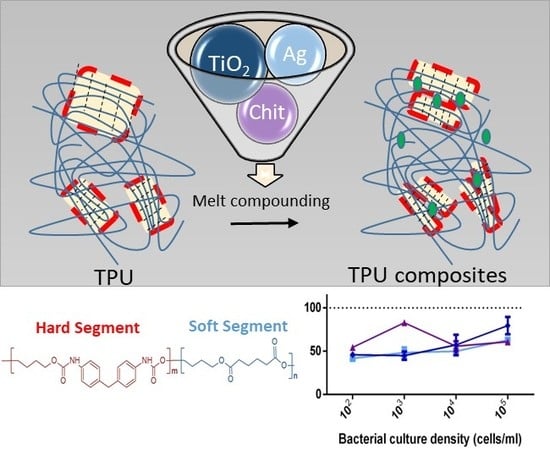
2.2. Polyurethane-Based Composite Preparation
2.3. Characterization
2.4. Antibacterial Tests
3. Results and Discussion
3.1. Preparation, Molecular, and Structural Characteristics
3.2. Thermal Analysis
3.3. Surface Morphology
3.4. Mechanical and Rheological Properties
3.5. Antibacterial Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

| Sample | Tc (°C) | Tm (°C) |
|---|---|---|
| TPU | 176 | 194 |
| TPU-Ag | 176 | 194 |
| TPU-Chit | 176 | 194 |
| TPU-TiO2 | 174 | 190 |
References
- Singha, P.; Locklin, J.; Handa, H.A. Review of the Recent Advances in Antimicrobial Coatings for Urinary Catheters. Acta Biomater. 2017, 50, 20–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaffaro, R.; Botta, L.; Gallo, G.; Puglia, A.M. Influence of Drawing on the Antimicrobial and Physical Properties of Chlorohexidine-Compounded Poly(caprolactone) Monofilaments. Macromol. Mater. Eng. 2015, 300, 1268–1277. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Sanfilippo, M.; Gallo, G.; Palazzolo, G.; Puglia, A.M. Combining in the melt physical and biological properties of poly(caprolactone) and chlorhexidine to obtain surgical monofilaments. Appl. Microbiol. Biotechnol. 2013, 97, 99–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, Y.; Garcia, C.V.; Ko, S.; Lee, W.; Shin, G.H.; Choi, J.C.; Park, S.-J.; Kima, J.T. Characterization and antibacterial properties of nanosilver-applied polyethylene and polypropylene composite films for food packaging applications. Food Biosci. 2018, 23, 83–90. [Google Scholar] [CrossRef]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Joost, U.; Juganson, K.; Visnapuu, M.; Mortimer, M.; Kahru, V.; Nõmmiste, E.; Joost, U.; Kisand, V.; Ivask, A. Photocatalytic antibacterial activity of nano-TiO2 (anatase)-based thin films: Effects on Escherichia coli cells and fatty acids. J. Photochem. Photobiol. B 2015, 142, 178–185. [Google Scholar] [CrossRef]
- Elbourne, A.; Crawford, R.J.; Ivanova, E.P. Nano-structured antimicrobial surfaces: From nature to synthetic analogues. J. Colloid Interface Sci. 2017, 508, 603–616. [Google Scholar] [CrossRef]
- Roe, D.; Karandikar, B.; Bonn-Savage, N.; Gibbins, B.; Roullet, J.-B. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J. Antimicrob. Chemother. 2008, 61, 869–876. [Google Scholar] [CrossRef]
- Rogachev, A.A.; Yarmolenko, M.A.; Rogachou, A.V.; Tapalski, D.V.; Liu, X.; Gorbachev, D.L. Morphology and structure of antibacterial nanocomposite organic–polymer and metal–polymer coatings deposited from active gas phase. RSC Adv. 2013, 3, 11226–11233. [Google Scholar] [CrossRef]
- Charpentier, P.A.; Burgess, K.; Wang, L.; Chowdhury, R.R.; Lotus, A.F.; Moula, G. Nano-TiO2/polyurethane composites for antibacterial and self-cleaning coatings. Nanotechnology 2012, 23, 425606–425615. [Google Scholar] [CrossRef]
- Mijnendonckx, K.; Leys, N.; Mahillon, J.; Silver, S.; Van Houdt, R. Antimicrobial silver: Uses, toxicity and potential for resistance. Biometals 2013, 26, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Modjarrad, K.; Ebnesajjad, S. Plastics Used in Medical Devices. In Handbook of Polymer Applications in Medicine and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Academic Press: San Diego, CA, USA, 2004. [Google Scholar]
- Alves, P.; Ferreira, P.; Gil, M.H. Biomedical Polyurethane-Based Materials. In Polyurethane: Properties, Structure and Applications; Nova Publishers: New York, NY, USA, 2012. [Google Scholar]
- Wang, H.-H.; Lin, M.-S. Biocidal Polyurethane and Its Antibacterial Properties. J. Polym. Res. 1998, 3, 177–186. [Google Scholar] [CrossRef]
- Davis, F.J.; Mitchell, G.R. Polyurethane Based Materials with Applications in Medical Devices. In Bio-Materials and Prototyping Applications in Medicine; Springer: New York, NY, USA, 2008. [Google Scholar]
- Xu, L.-C.; Siedlecki, C.A. Antibacterial polyurethanes. Adv. Polyurethane Biomater. 2016, 9, 247–284. [Google Scholar]
- Zilberman, M.; Elsner, J.J. Antibiotic-eluting medical devices for various applications. J. Control. Release 2008, 130, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.B.; Cooper, S.L. Morphology and properties of segmented polyether polyurethaneureas. Macromolecules 1983, 16, 775–786. [Google Scholar] [CrossRef]
- Li, Y.; Gao, T.; Liu, J.; Linliu, K.; Desper, C.R.; Chu, B. Multiphase structure of a segmented polyurethane: Effects of temperature and annealing. Macromolecules 1992, 25, 7365–7372. [Google Scholar] [CrossRef]
- Sui, T.; Baimpas, N.; Dolbnya, I.P.; Prisacariu, C.; Korsunsky, A.M. Multiple-length-scale deformation analysis in a thermoplastic polyurethane. Nat. Commun. 2015, 6, 6583–6592. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.H.; Chou, N.K.; Wu, W.J.; Hsu, S.H.; Whu, S.W.; Ho, G.H.; Tsai, C.L.; Wang, S.S.; Chu, S.H.; Hsieh, K.H. Physical Properties of Water-Borne Polyurethane blended with Chitosan. J. Appl. Polym. Sci. 2007, 104, 2683–2689. [Google Scholar] [CrossRef]
- Van den Broek, L.A.M.; Knoop, R.J.I.; Kappen, F.H.J.; Boeriu, C.J. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef]
- Kucinska-Lipka, J.; Gubanska, I.; Janik, H. Polyurethanes modified with natural polymers for medical application. Polimery 2013, 58, 678–684. [Google Scholar] [CrossRef]
- Silva, S.S.; Menezes, S.M.C.; Garcia, B.R. Synthesis and characterization of polyurethane-g-chitosan. Eur. Polym. J. 2003, 39, 1515–1519. [Google Scholar] [CrossRef]
- Francolini, I.; D’Ilario, L.; Guaglianone, E.; Donelli, G.; Martinelli, A.; Piozzi, A. Polyurethane anionomers containing metal ions with antimicrobial properties: Thermal, mechanical and biological characterization. Acta Biomater. 2010, 6, 3482–3490. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, H.; Yeganeh, H.; Mehdipour-Ataei, S.; Shokrgozar, M.A.; Yari, A.; Saeedi-Eslami, S.N. Synthesis and characterization of antibacterial polyurethane coatings from quaternary ammonium salts functionalized soybean oil based polyols. Mater. Sci. Eng. C 2013, 33, 153–164. [Google Scholar] [CrossRef]
- Wang, C.-H.; Hou, G.-G.; Du, Z.-Z.; Cong, W.; Sun, J.-F.; Xu, Y.-Y.; Liu, W.-S. Synthesis, characterization and antibacterial properties of polyurethane material functionalized with quaternary ammonium salt. Polym. J. 2016, 48, 259–265. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Bertoglio, F.; Owczarek, J.S.; Bruni, G.; Kozanecki, M.; Kenny, J.M.; Torre, L.; Visai, L.; Puglia, D. Polyvinyl alcohol/chitosan hydrogels with enhanced antioxidant and antibacterial properties induced by lignin nanoparticles. Carbohydr. Polym. 2018, 181, 275–284. [Google Scholar] [CrossRef]
- Bertoglio, F.; Bloise, N.; Oriano, M.; Petrini, P.; Sprio, S.; Imbriani, M.; Tampieri, A.; Visai, L. Treatment of biofilm communities: An update on new tools from the nanosized world. Appl. Sci. 2018, 8, 845. [Google Scholar] [CrossRef] [Green Version]
- Pallavicini, P.; Arciola, C.R.; Bertoglio, F.; Curtosi, S.; Dacarro, G.; D’Agostino, A.; Ferrari, F.; Merli, D.; Milanese, C.; Rossi, S. Silver nanoparticles synthesized and coated with pectin: An ideal compromise for anti-bacterial and anti-biofilm action combined with wound-healing properties. J. Colloid Interface Sci. 2017, 498, 271–281. [Google Scholar] [CrossRef]
- Yalcinkaya, E.E.; Puglia, D.; Fortunati, E.; Bertoglio, F.; Bruni, G.; Visai, L.; Kenny, J.M. Cellulose nanocrystals as templates for cetyltrimethylammonium bromide mediated synthesis of Ag nanoparticles and their novel use in PLA films. Carbohydr. Polym. 2017, 157, 1557–1567. [Google Scholar] [CrossRef]
- Oprea, S.; Oprea, V. Mechanical behavior during different weathering tests of the polyurethane elastomers films. Eur. Polym. J. 2002, 38, 1205–1210. [Google Scholar] [CrossRef]
- Stribeck, A.; Pöselt, E.; Eling, B.; Jokari-Sheshdeha, F.; Hoell, A. Thermoplastic polyurethanes with varying hard-segment components. Mechanical performance and a filler-crosslink conversion of hard domains as monitored by SAXS. Eur. Polym. J. 2017, 94, 340–353. [Google Scholar] [CrossRef]
- Lempesis, N.; in‘t Veld, P.J.; Rutledge, G.C. Simulation of the structure and mechanics of crystalline 4,4′-diphenylmethane diisocyanate (MDI) with n-butanediol (BDO) as chain extender. Polymer 2016, 107, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Blackwell, J.; Nagarajan, M.R.; Hoitink, T.B. Structure of polyurethane elastomers. X-ray diffraction and conformational analysis of MDI-propandiol and MDI-ethylene glycol hard segments. Polymer 1981, 22, 1003–1008. [Google Scholar] [CrossRef]
- Bonart, R.; Muller, E.H. Phase separation in urethane elastomers as judged by low-angle X-ray scattering. I. Fundamentals. J. Macromol. Sci. Phys. 1974, 10, 177–189. [Google Scholar] [CrossRef]
- Bonart, R.; Muller, E.H. Phase separation in urethane elastomers as judged by low-angle X-ray scattering. II. Experimental Results. J. Macromol. Sci. Phys. 1974, 10, 345–357. [Google Scholar] [CrossRef]
- Tian, Q.; Krakovský, I.; Yan, G.; Bai, L.; Liu, J.; Sun, G.; Rosta, L.; Chen, B.; Almásy, L. Microstructure Changes in Polyester Polyurethane upon Thermal and Humid Aging. Polymers 2016, 8, 197. [Google Scholar] [CrossRef] [Green Version]
- Herrera, M.; Matuschek, G.; Kettrup, A. Thermal degradation of thermoplastic polyurethane elastomers (TPU) based on MDI. Polym. Degrad. Stab. 2002, 78, 323–331. [Google Scholar] [CrossRef]
- Tabuani, D.; Bellucci, F.; Terenzi, A.; Camino, G. Flame retarded Thermoplastic Polyurethane (TPU) for cable jacketing application. Polym. Degrad. Stab. 2012, 97, 2594–2601. [Google Scholar] [CrossRef]
- Leibler, L. Theory of Microphase Separation in Block Copolymers. Macromolecules 1980, 13, 1602–1617. [Google Scholar] [CrossRef]
- Villani, M.; Scheerder, J.; van Benthem, R.A.T.M.; de With, G. Interfacial interactions of poly(urethane–urea) based primers with polypropylene. Eur. Polym. J. 2014, 56, 118–130. [Google Scholar] [CrossRef]
- Seymour, R.W.; Cooper, S.L. Thermal Analysis of Polyurethane Block Polymers. Macromolecules 1973, 6, 48–53. [Google Scholar] [CrossRef]
- Paik Sung, C.S.; Schneider, N.S.J. Structure-property relationships of polyurethanes based on toluene di-isocyanate. Mater. Sci. 1978, 13, 1689–1699. [Google Scholar] [CrossRef]
- Leung, L.M.; Koberstein, J.T. DSC Annealing Study of Microphase Separation and Multiple Endothermic Behavior in Polyether-Based Polyurethane Block Copolymers. Macromolecules 1986, 19, 706–713. [Google Scholar] [CrossRef]
- Yoon, P.Y.; Han, C.D. Effect of Thermal History on the Rheological Behavior of Thermoplastic Polyurethanes. Macromolecules 2000, 33, 2171–2183. [Google Scholar] [CrossRef]
- Bates, F.S. Block Copolymers near the Microphase Separation Transition. 2. Linear Dynamic Mechanical Properties. Macromolecules 1984, 17, 2607–2613. [Google Scholar] [CrossRef]
- Han, C.D.; Baek, D.M.; Kim, J.K.; Ogawa, T.; Sakamoto, N.; Hashimoto, T. Effect of Volume Fraction on the Order-Disorder Transition in Low Molecular Weight Polystyrene-block-Polyisoprene Copolymers. 1. Order-Disorder Transition Temperature Determined by Rheological Measurements. Macromolecules 1995, 28, 5043–5062. [Google Scholar] [CrossRef]
- Chambon, F.; Petrovic, Z.S.; MacKnight, W.J.; Winter, H.H. Rheology of Model Polyurethanes at the Gel Point. Macromolecules 1986, 19, 2146–2149. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, S.; Nishiguchi, D.; Kojio, K.; Furukawa, M. Effects of aggregation structure on rheological properties of thermoplastic polyurethanes. Polymer 2007, 48, 4793–4803. [Google Scholar] [CrossRef]
- Kim, S.S.; Han, C.D. Oscillatory shear flow behavior of a thermotropic liquid-crystalline polymer. Polymer 1994, 35, 93–103. [Google Scholar] [CrossRef]
- Cossar, S.; Nichetti, D.; Grizzuti, N. A rheological study of the phase transition in thermoplastic polyurethanes. Critical gel behavior and microstructure development. J. Rheol. 2004, 48, 691–703. [Google Scholar] [CrossRef]
- Mourier, E.; Fulchiron, R.; Mechin, F. Shear-Induced Structuring Kinetics in Thermoplastic Segmented Polyurethanes Monitored by Rheological Tools. J. Polym. Sci. Part B: Polym. Phys. 2010, 48, 190–201. [Google Scholar] [CrossRef]
- Aurilia, M.; Piscitelli, F.; Sorrentino, L.; Lavorgna, M.; Iannace, S. Detailed analysis of dynamic mechanical properties of TPU nanocomposite: The role of the interfaces. Eur. Polym. J. 2011, 47, 925–936. [Google Scholar] [CrossRef]
- Abraham, J.; Sharika, T.; George, S.C.; Thomas, S. Rheological Percolation in Thermoplastic Polymer Nanocomposites. Rheol.: Open Access 2017, 1, 102–117. [Google Scholar]
- Seymour, R.W.; Estes, G.M.; Cooper, S.L. Infrared studies of segmented polyurethane elastomers. 1. Hydrogen bonding. Macromolecules 1970, 3, 579–583. [Google Scholar] [CrossRef]
- Treolar, L.R.G. The Physics of Rubber Elasticity; Claredon Press: Oxford, UK, 1975. [Google Scholar]
- Coppola, S.; Acierno, S.; Grizzuti, N.; Vlassopoulos, D. Viscoelastic Behavior of Semicrystalline Thermoplastic Polymers during the Early Stages of Crystallization. Macromolecules 2006, 39, 1507–1514. [Google Scholar] [CrossRef]
- Boutahar, K.; Carrot, C.; Guillet, J. Crystallization of Polyolefins from Rheological Measurements-Relation between the Transformed Fraction and the Dynamic Moduli. Macromolecules 1998, 31, 1921–1929. [Google Scholar] [CrossRef]
- Lin, Y.G.; Mallín, D.T.; Chien, J.C.W.; Winter, H. Dynamic Mechanical Measurement of Crystallization-Induced Gelation in Thermoplastic Elastomeric Poly(propylene). Macromolecules 1991, 24, 850–854. [Google Scholar] [CrossRef]
- Balzano, L.; Rastogi, S.; Peters, G.W.M. Flow Induced Crystallization in Isotactic Polypropylene-1,3:2,4-Bis(3,4-dimethylbenzylidene)sorbitol Blends: Implications on Morphology of Shear and Phase Separation. Macromolecules 2008, 41, 399–408. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [Green Version]














| Sample | Mw (Kg/mol) | Mn (Kg/mol) | Mw/Mn | T2% (°C) | T50% (°C) |
|---|---|---|---|---|---|
| TPU unprocessed | 94.8 | 52.5 | 1.8 | ||
| TPU | 80.7 | 47.3 | 1.7 | 309 | 386 |
| TPU-Ag | 76.7 | 45.0 | 1.7 | 305 | 385 |
| TPU-Chit | 77.6 | 45.4 | 1.7 | 305 | 379 |
| TPU-TiO2 | 51.0 | 33.8 | 1.5 | 279 | 383 |
| Sample | E (MPa) | σmax (MPa) | εbreak (%) |
|---|---|---|---|
| TPU | 26.2 ± 1.4 | 36.4 ± 1.6 | 1075 ± 44 |
| TPU-Ag | 30.5 ± 1.6 | 26.4 ± 3.1 | 975 ± 87 |
| TPU-chitosan | 33.9 ± 1.5 | 25.9 ± 1.3 | 845 ± 21 |
| TPU-TiO2 | 19.8 ± 4.7 | 4.4 ± 1.1 | 123 ± 45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villani, M.; Consonni, R.; Canetti, M.; Bertoglio, F.; Iervese, S.; Bruni, G.; Visai, L.; Iannace, S.; Bertini, F. Polyurethane-Based Composites: Effects of Antibacterial Fillers on the Physical-Mechanical Behavior of Thermoplastic Polyurethanes. Polymers 2020, 12, 362. https://doi.org/10.3390/polym12020362
Villani M, Consonni R, Canetti M, Bertoglio F, Iervese S, Bruni G, Visai L, Iannace S, Bertini F. Polyurethane-Based Composites: Effects of Antibacterial Fillers on the Physical-Mechanical Behavior of Thermoplastic Polyurethanes. Polymers. 2020; 12(2):362. https://doi.org/10.3390/polym12020362
Chicago/Turabian StyleVillani, Maurizio, Roberto Consonni, Maurizio Canetti, Federico Bertoglio, Stefano Iervese, Giovanna Bruni, Livia Visai, Salvatore Iannace, and Fabio Bertini. 2020. "Polyurethane-Based Composites: Effects of Antibacterial Fillers on the Physical-Mechanical Behavior of Thermoplastic Polyurethanes" Polymers 12, no. 2: 362. https://doi.org/10.3390/polym12020362
APA StyleVillani, M., Consonni, R., Canetti, M., Bertoglio, F., Iervese, S., Bruni, G., Visai, L., Iannace, S., & Bertini, F. (2020). Polyurethane-Based Composites: Effects of Antibacterial Fillers on the Physical-Mechanical Behavior of Thermoplastic Polyurethanes. Polymers, 12(2), 362. https://doi.org/10.3390/polym12020362