Flame Retardation of Natural Rubber: Strategy and Recent Progress
Abstract
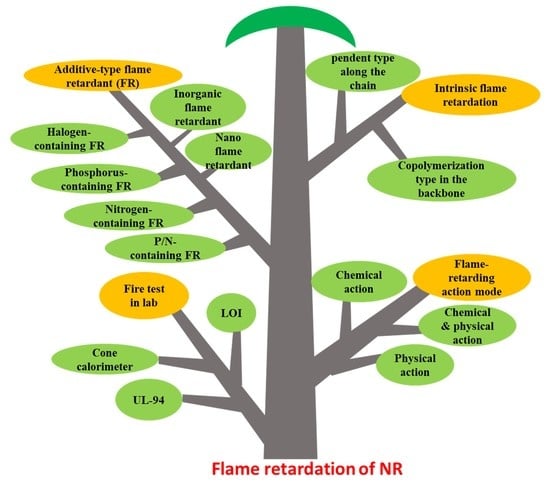
:1. Introduction
2. Thermal Decomposition and Burning Behavior of NR
2.1. Thermal Decomposition of NR
2.2. The Burning Behavior of NR
3. Laboratory Fire Test
3.1. UL 94 Vertical Flammability Standard Test
3.2. Limiting Oxygen Index
3.3. Cone Calorimeter
- (1)
- Time to ignition (TTI, s): under the same irradiation power and sample thickness, the longer TTI means it is harder to ignite the material. For flame-retarding polymers, they often decompose in advance due to the addition of flame retardant, thereby shortening the TTI. Therefore, the shorter TTI does not mean that the flame retardancy of materials becomes worse.
- (2)
- Heat release rate (HRR, KW/m2): it is defined as the heat release per unit time and unit surface area during the cone calorimetry test. Particularly, the peak value of HRR (PHRR) or its maximum (HRRmax) is used to evaluate the fire performance of materials.
- (3)
- Total heat release (THR, kJ/m2): the total calorific value released per unit area after the combustion process for materials, which can be calculated according to the integration of the HRR vs. time.
- (4)
- Fire growth rate (FGR, KW/(s·m2)): the FGR is expressed as FGR = PHRR/tPHRR [18], tPHRR is the time to reach the PHRR. The lower the FGR value is, the better the fire performance of the material is.
- (5)
- Mass loss rate (MLR, g/s) is the mass loss of material per unit time during burning.
- (6)
- Smoke product rate (SPR, m2/s) and total smoke production (TSP, m2) reflect the combustion degree of materials.
4. The Flame-Retarding Action Mode
4.1. Physical Action
4.2. Chemical Action
5. Flame Retardance of NR
5.1. Intrinsic Flame Retardation
5.2. Additive-Type Flame Retardation
5.2.1. Halogen-Containing Flame Retardants
5.2.2. Phosphorus-Containing Flame Retardants
5.2.3. Nitrogen-Containing Flame Retardants
5.2.4. Phosphorus/Nitrogen Flame Retardants
5.2.5. Inorganic Flame Retardants
5.2.6. Nano Flame Retardants
6. Concluding Remarks and Outlook
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Dong, X.; Hao, W.; Yi, Z.; Xi, G.; Ding, W. Application properties of TCP/OMMT flame-retardant system in NR composites. J. Elastomers Plast. 2013, 45, 107–119. [Google Scholar] [CrossRef]
- Kind, D.J.; Hull, T.R. A review of candidate fire retardants for polyisoprene. Polym. Degrad. Stab. 2012, 97, 201–213. [Google Scholar] [CrossRef]
- Derouet, D.; Radhakrishnan, N.; Brosse, J.C.; Boccaccio, G. Phosphorus modification of epoxidized liquid natural-rubber to improve flame resistance of vulcanized rubbers. J. Appl. Polym. Sci. 1994, 52, 1309–1316. [Google Scholar] [CrossRef]
- Carli, L.N.; Roncato, C.R.; Zanchet, A.; Mauler, R.S.; Giovanela, M.; Brandalise, R.N.; Crespo, J.S. Characterization of natural rubber nanocomposites filled with organoclay as a substitute for silica obtained by the conventional two-roll mill method. Appl. Clay Sci. 2011, 52, 56–61. [Google Scholar] [CrossRef]
- Huang, G.B.; Li, Y.J.; Han, L.A.; Gao, J.R.; Wang, X. A novel intumescent flame retardant-functionalized montmorillonite: Preparation, characterization, and flammability properties. Appl. Clay Sci. 2011, 51, 360–365. [Google Scholar] [CrossRef]
- Wang, D.L.; Liu, Y.; Wang, D.Y.; Zhao, C.X.; Mou, Y.R.; Wang, Y.Z. A novel intumescent flame-retardant system containing metal chelates for polyvinyl alcohol. Polym. Degrad. Stab. 2007, 92, 1555–1564. [Google Scholar] [CrossRef]
- Williams, C.G.H., IV. On isoprene and caoutchine. Proc. R. Soc. Lond. 1860, 10, 516–519. [Google Scholar]
- Colin, X.; Audouin, L.; Verdu, J. Kinetic modelling of the thermal oxidation of polyisoprene elastomers. Part 1: Unvulcanized unstabilized polyisoprene. Polym. Degrad. Stab. 2007, 92, 886–897. [Google Scholar] [CrossRef]
- Midgley, T.; Henne, A.L. Natural and synthetic rubber I Products of the destructive distillation of natural rubber. J. Am. Chem. Soc. 1929, 51, 1215–1226. [Google Scholar] [CrossRef]
- Bolland, J.L.; Orr, W.J.C. Thermal breakdown of rubber. Rubber Chem. Technol. 1946, 19, 277–282. [Google Scholar] [CrossRef]
- Straus, S.; Madorsky, S.L. Thermal degradation of unvulcanized and vulcanized rubber in a vacuum. Ind. Eng. Chem. 1956, 48, 1212–1219. [Google Scholar] [CrossRef]
- Chen, F.Z.; Qian, J.L. Studies on the thermal degradation of cis-1,4-polyisoprene. Fuel 2002, 81, 2071–2077. [Google Scholar] [CrossRef]
- Reshetnikov, S.M.; Reshetnikov, I.S. Oxidation kinetic of volatile polymer degradation products. Polym. Degrad. Stab. 1999, 64, 379–385. [Google Scholar] [CrossRef]
- Cataldo, F. Thermal depolymerization and pyrolysis of cis-1,4-polyisoprene: Preparation of liquid polyisoprene and terpene resin. J. Anal. Appl. Pyrolysis 1998, 44, 121–130. [Google Scholar] [CrossRef]
- Neiman, M.B. Mechanism of the oxidative thermal degradation and of the stabilisation of polymers. Russ. Chem. Rev. 1964, 33, 13–27. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Huggett, C. Estimation of rate of heat release by means of oxygen-consumption measurements. Fire Mater. 1980, 4, 61–65. [Google Scholar] [CrossRef]
- Breulet, H.; Steenhuizen, T. Fire testing of cables: Comparison of SBI with FIPEC/Europacable tests. Polym. Degrad. Stab. 2005, 88, 150–158. [Google Scholar] [CrossRef]
- Hollingbery, L.A.; Hull, T.R. The fire retardant behaviour of huntite and hydromagnesite—A review. Polym. Degrad. Stab. 2010, 95, 2213–2225. [Google Scholar] [CrossRef] [Green Version]
- Hull, T.R.; Witkowski, A.; Hollingbery, L. Fire retardant action of mineral fillers. Polym. Degrad. Stab. 2011, 96, 1462–1469. [Google Scholar] [CrossRef] [Green Version]
- Intharapat, P.; Derouet, D.; Nakason, C. Thermal and flame resistance properties of natural rubber-g-poly(dimethyl(methacryloyloxymethyl)phosphonate). J. Appl. Polym. Sci. 2010, 115, 255–262. [Google Scholar] [CrossRef]
- Intharapat, P.; Nakason, C.; Kongnoo, A. Preparation of boric acid supported natural rubber as a reactive flame retardant and its properties. Polym. Degrad. Stab. 2016, 128, 217–227. [Google Scholar] [CrossRef]
- Baysal, E.; Yalinkilic, M.K. A comparative study on stability and decay resistance of some environmentally friendly fire-retardant boron compounds. Wood Sci. Technol. 2005, 39, 169–186. [Google Scholar] [CrossRef]
- Kokklin, K.; Tangpasuthadol, V.; Bhanthumnavin, W. Grafting of phosphonate monomer onto natural rubber latexes via emulsion polymerization. Adv. Mater. Res. 2010, 93, 125–128. [Google Scholar] [CrossRef]
- Lu, S.Y.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Troitzsch, J.H. Overview of flame retardants. Chim. Oggi-Chem. Today 1998, 16, 18–24. [Google Scholar]
- Trexler, H.E. The formulation of nonburning elastomer compounds. Rubber Chem. Technol. 1973, 46, 1114–1125. [Google Scholar] [CrossRef]
- Menon, A.R.R. Stress-relaxation characteristics of natural rubber modified with phosphorylated cashew nut shell liquid prepolymer. J. Appl. Polym. Sci. 1997, 65, 2183–2189. [Google Scholar] [CrossRef]
- Menon, A.R.R. Flame-retardant characteristics of natural rubber modified with a bromo derivative of phosphorylated cashew nut shell liquid. J. Fire Sci. 1997, 15, 3–13. [Google Scholar] [CrossRef]
- Ismawi, D.H.A.; Harper, J.F.; Ansarifar, A. Influence of flame retardant additives on the flammability behaviour of natural rubber (NR). J. Rubber Res. 2008, 11, 223–236. [Google Scholar]
- Yang, A.-H.; Deng, C.; Chen, H.; Wei, Y.-X.; Wang, Y.-Z. A novel Schiff-base polyphosphate ester: Highly-efficient flame retardant for polyurethane elastomer. Polym. Degrad. Stab. 2017, 144, 70–82. [Google Scholar] [CrossRef]
- Dong, L.-P.; Deng, C.; Li, R.-M.; Cao, Z.J.; Lin, L.; Chen, L.; Wang, Y.Z. Poly(piperazinyl phosphamide): A novel highly-efficient charring agent for an EVA/APP intumescent flame retardant system. RSC Adv. 2016, 6, 30436–30444. [Google Scholar] [CrossRef]
- Menon, A.R.R.; Pillai, C.K.S.; Nando, G.B. Modification of natural rubber with phosphatic plasticizers: A comparison of phosphorylated cashew nut shell liquid prepolymer with 2-ethyl hexyl diphenyl phosphate. Eur. Polym. J. 1998, 34, 923–929. [Google Scholar] [CrossRef]
- Nelson, G.L. (Ed.) Fire and Polymers: Hazards Identification and Prevention; American Chemical Society: Washington, DC, USA, 1990. [Google Scholar]
- Liu, G.; Zhao, J.; Zhang, Y.; Liu, S.; Ye, H. Synthesis and application in polypropylene of a novel nitrogen-containing intumescent flame-retardant. Polym. Polym. Compos. 2007, 15, 191–198. [Google Scholar] [CrossRef]
- Horacek, H.; Grabner, R. Advantages of flame retardants based on nitrogen compounds. Polym. Degrad. Stab. 1996, 54, 205–215. [Google Scholar] [CrossRef]
- Dufton, P.W. Additives and Materials; iSmithers Rapra Publishing: Akron, OH, USA, 1995. [Google Scholar]
- Bourbigot, S.; Le Bras, M.; Duquesne, S.; Rochery, M. Recent advances for intumescent polymers. Macromol. Mater. Eng. 2004, 289, 499–511. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.-Y.; Wang, J.-S.; Song, Y.-P.; Wang, Y.-Z. A novel intumescent flame-retardant LDPE system and its thermo-oxidative degradation and flame-retardant mechanisms. Polym. Adv. Technol. 2008, 19, 1566–1575. [Google Scholar] [CrossRef]
- Shao, Z.-B.; Deng, C.; Tan, Y.; Chen, M.-J.; Chen, L.; Wang, Y.-Z. An efficient mono-component polymeric intumescent flame retardant for polypropylene: Preparation and application. ACS Appl. Mater. Interfaces 2014, 6, 7363–7370. [Google Scholar] [CrossRef]
- Shao, Z.-B.; Deng, C.; Tan, Y.; Chen, M.-J.; Chen, L.; Wang, Y.-Z. Flame retardation of polypropylene via a novel intumescent flame retardant: Ethylenediamine-modified ammonium polyphosphate. Polym. Degrad. Stab. 2014, 106, 88–96. [Google Scholar] [CrossRef]
- Shao, Z.-B.; Deng, C.; Tan, Y.; Yu, L.; Chen, M.J.; Chen, L.; Wang, Y.Z. Ammonium polyphosphate chemically-modified with ethanolamine as an efficient intumescent flame retardant for polypropylene. J. Mater. Chem. A 2014, 2, 13955–13965. [Google Scholar] [CrossRef]
- Yan, Y.-W.; Chen, L.; Jian, R.-K.; Kong, S.; Wang, Y.-Z. Intumescence: An effect way to flame retardance and smoke suppression for polystryene. Polym. Degrad. Stab. 2012, 97, 1423–1431. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y. Synthesis of an intumescent flame retardant (IFR) agent and application in a natural rubber (NR) system. J. Elastomers Plast. 2007, 39, 33–51. [Google Scholar]
- Wang, J.; Guo, Y. Hyperbranched intumescent flame-retardant agent: Application to natural rubber composites. J. Appl. Polym. Sci. 2011, 122, 3474–3482. [Google Scholar]
- Wang, J.; Chen, Y. Effect of microencapsulation and 4A zeolite on the properties of intumescent flame-retardant natural rubber composites. J. Fire Sci. 2008, 26, 153–171. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y. Microencapsulation of intumescent flame-retardant agent: Application to flame-retardant natural rubber composite. J. Appl. Polym. Sci. 2007, 104, 1828–1838. [Google Scholar] [CrossRef]
- Wang, N.; Mi, L.; Wu, Y.; Zhang, J.; Fang, Q. Double-layered co-microencapsulated ammonium polyphosphate and mesoporous MCM-41 in intumescent flame-retardant natural rubber composites. J. Therm. Anal. Calorim. 2014, 115, 1173–1181. [Google Scholar] [CrossRef]
- Wang, N.; Wu, Y.; Mi, L.; Zhang, J.; Li, X.; Fang, Q. The influence of silicone shell on double-layered microcapsules in intumescent flame-retardant natural rubber composites. J. Therm. Anal. Calorim. 2014, 118, 349–357. [Google Scholar] [CrossRef]
- Wang, N.; Xu, G.; Wu, Y.; Zhang, J.; Hu, L.; Luan, H.; Fang, Q. The influence of expandable graphite on double-layered microcapsules in intumescent flame-retardant natural rubber composites. J. Therm. Anal. Calorim. 2016, 123, 1239–1251. [Google Scholar] [CrossRef]
- Wang, J.; Yang, K.; Zheng, X. Studies on the effect of 4A zeolite on the properties of intumescent flame-retardant agent filled natural rubber composites. J. Polym. Res. 2009, 16, 427–436. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, M.; Kang, P.; Zhang, J.; Fang, Q.; Li, W. Synergistic effect of graphene oxide and mesoporous structure on flame retardancy of nature rubber/IFR composites. Materials 2018, 11, 1005. [Google Scholar] [CrossRef] [Green Version]
- Fu, M.Z.; Qu, B.J. Synergistic flame retardant mechanism of fumed silica in ethylene-vinyl acetate/magnesium hydroxide blends. Polym. Degrad. Stab. 2004, 85, 633–639. [Google Scholar] [CrossRef]
- Ye, L.; Wu, Q.; Qu, B. Synergistic effects and mechanism of multiwalled carbon nanotubes with magnesium hydroxide in halogen-free flame retardant EVA/MH/MWNT nanocomposites. Polym. Degrad. Stab. 2009, 94, 751–756. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.L.; Chen, X.L.; Jiao, C.M. Synergistic effect between hollow glass beads and aluminium hydroxide in flame retardant EVA composites. Plast. Rubber Compos. 2014, 43, 77–81. [Google Scholar] [CrossRef]
- Beyer, G. Flame retardant properties of EVA-nanocomposites and improvements by combination of nanofillers with aluminium trihydrate. Fire Mater. 2001, 25, 193–197. [Google Scholar] [CrossRef]
- Morgan, A.B.; Gilman, J.W. An overview of flame retardancy of polymeric materials: Application, technology, and future directions. Fire Mater. 2013, 37, 259–279. [Google Scholar] [CrossRef] [Green Version]
- Sabet, M.; Hassan, A.; Ratnam, C.T. Flammability and thermal characterization of aluminum hydroxide filled with LDPE. Int. Polym. Process. 2013, 28, 393–397. [Google Scholar] [CrossRef]
- Sonnier, R.; Viretto, A.; Dumazert, L.; Longerey, M.; Buonomo, S.; Gallard, B.; Longuet, C.; Cavodeau, F.; Lamy, R.; Freitag, A. Fire retardant benefits of combining aluminum hydroxide and silica in ethylene-vinyl acetate copolymer (EVA). Polym. Degrad. Stab. 2016, 128, 228–236. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.Z.; Zhou, Q.; Shao, W. Aluminum hydroxide filled ethylene vinyl acetate (EVA) composites: Effect of the interfacial compatibilizer and the particle size. J. Mater. Sci. 2007, 42, 4227–4232. [Google Scholar] [CrossRef]
- Zhang, X.G.; Guo, F.; Chen, J.F.; Wang, G.Q.; Liu, H. Investigation of interfacial modification for flame retardant ethylene vinyl acetate copolymer/alumina trihydrate nanocomposites. Polym. Degrad. Stab. 2005, 87, 411–418. [Google Scholar] [CrossRef]
- Canaud, C.; Visconte, L.U.Y.; Nunes, R.C.R. Mechanical and flammability properties of ATH-filled EPDM compositions. Macromol. Mater. Eng. 2001, 286, 377–381. [Google Scholar] [CrossRef]
- Nachtigall, S.M.B.; Miotto, M.; Schneider, E.E.; Mauler, R.S.; Forte, M.M.C. Macromolecular coupling agents for flame retardant materials. Eur. Polym. J. 2006, 42, 990–999. [Google Scholar] [CrossRef]
- Plentz, R.S.; Miotto, M.; Schneider, E.E.; Forte, M.S.M.C.; Mauler, R.S.; Nachtigall, S.M.B. Effect of a macromolecular coupling agent on the properties of aluminum hydroxide/PP composites. J. Appl. Polym. Sci. 2006, 101, 1799–1805. [Google Scholar] [CrossRef]
- WE, H. Inorganic Hydroxides and Hydroxycarbonates: Their Function and Use as Flame-Retardant Additives; Marcel Dekker Inc.: New York, NY, USA, 2000; pp. 285–352. [Google Scholar]
- ITRI. Zinc Stannates in Halogen-Free Polymer Formulations; Technical Bulletin No. 2; Bethesda: Rockville, MD, USA, 1992. [Google Scholar]
- Myers, R.E.; Dickens, E.D.; Licursi, E.; Evans, R.E. Ammonium pentaborate—An intumescent flame-retardant for thermoplastic polyurethanes. J. Fire Sci. 1985, 3, 432–449. [Google Scholar] [CrossRef]
- Lopattananon, N.; Walong, A.; Kaesaman, A.; Seadan, M. Effect of MAH-g-PP on the performance of ATH filled NR/PP thermoplastic vulcanisates. J. Rubber Res. 2016, 19, 243–260. [Google Scholar]
- Chen, S.; Zhang, Y.; Wang, R.; Yu, H.; Hoch, M.; Gu, S. Mechanical properties, flame retardancy, hot-air ageing, and hot-oil ageing resistance of ethylene-vinyl acetate rubber/hydrogenated nitrile-butadiene rubber/magnesium hydroxide composites. J. Appl. Polym. Sci. 2009, 114, 3310–3318. [Google Scholar] [CrossRef]
- Ma, H.; Song, P.; Fang, Z. Flame retarded polymer nanocomposites: Development, trend and future perspective. Sci. China Chem. 2011, 54, 302–313. [Google Scholar] [CrossRef]
- Wei, Y.-X.; Deng, C.; Chen, H.; Wan, L.; Wei, W.-C.; Wang, Y.-Z. Novel core-shell hybrid nanosphere towards the mechanical enhancement and fire retardance of polycarbonate. ACS Appl. Mater. Interfaces 2018, 10, 28036–28050. [Google Scholar] [CrossRef]
- Wei, Y.-X.; Deng, C.; Zhao, Z.-Y.; Wang, Y.-Z. A novel organic-inorganic hybrid SiO2@DPP for the fire retardance of polycarbonate. Polym. Degrad. Stab. 2018, 154, 177–185. [Google Scholar] [CrossRef]
- Fujiwara, S.; Sakamoto, K. Falmmability Properties of Nylon-6/Mica Nanocomposites. Japan Kokai Patent Application No. SHO511976-109998, 29 September 1976. [Google Scholar]
- Dai, K.; Sun, S.; Xu, W.; Song, Y.; Deng, Z.; Qian, X. Covalently-functionalized graphene oxide via introduction of bifunctional phosphorus-containing molecules as an effective flame retardant for polystyrene. RSC Adv. 2018, 8, 24993–25000. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Bao, C.; Song, L.; Yuan, B.; Hu, Y. In situ polymerization of graphene, graphite oxide, and functionalized graphite oxide into epoxy resin and comparison study of on-the-flame behavior. Ind. Eng. Chem. Res. 2011, 50, 7772–7783. [Google Scholar] [CrossRef]
- Du, J.-Z.; Jin, L.; Zeng, H.-Y.; Feng, B.; Xu, S.; Zhou, E.G.; Shi, X.K.; Liu, L.; Hu, X. Facile preparation of an efficient flame retardant and its application in ethylene vinyl acetate. Appl. Clay Sci. 2019, 168, 96–105. [Google Scholar] [CrossRef]
- Kausar, A.; Rafique, I.; Muhammad, B. Significance of carbon nanotube in flame-retardant polymer/CNT composite: A review. Polym. Plast. Technol. Eng. 2017, 56, 470–487. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, W.; He, X.; Yang, R. High-efficiency flame retardency of epoxy resin composites with perfect T-8 caged phosphorus containing polyhedral oligomeric silsesquioxanes (P-POSSs). Compos. Sci. Technol. 2016, 127, 8–19. [Google Scholar] [CrossRef]
- Zhou, X.; Ran, S.; Hu, H.; Fang, Z. Improving flame-retardant efficiency by incorporation of fullerene in styrene-butadiene-styrene block copolymer/aluminum hydroxide composites. J. Therm. Anal. Calorim. 2016, 125, 199–204. [Google Scholar] [CrossRef]
- Bee, S.-L.; Abdullah, M.A.A.; Bee, S.-T.; Sin, L.T.; Rahmat, A.R. Polymer nanocomposites based on silylated-montmorillonite: A review. Prog. Polym. Sci. 2018, 85, 57–82. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J. Natural rubber/dendrimer modified montmorillonite nanocomposites: Mechanical and flame-retardant properties. Materials 2018, 11, 41. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Wang, J. Preparation of novel flame-retardant organoclay and its application to natural rubber composites. J. Phys. Chem. Solids 2018, 115, 137–147. [Google Scholar] [CrossRef]
- Amarasiri, A.; Ratnayake, U.N.; De Silva, U.K.; Walpalage, S.; Siriwardene, S. Natural rubber latex-clay nanocomposite: Use of montmorillonite clay as an alternative for conventional CaCO3. J. Natl. Sci. Found. Sri Lanka 2013, 41, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Guo, X.; Zheng, X.; Zhao, Y.; Li, W. Enhanced flame-retardant capacity of natural rubber/organo-montmorillonite and hyper-branched organo-montmorillonite composites. Clays Clay Miner. 2011, 59, 446–458. [Google Scholar] [CrossRef]
- Zhu, T.T.; Zhou, C.H.; Kabwe, F.B.; Wu, Q.Q.; Li, C.S.; Zhang, J.R. Exfoliation of montmorillonite and related properties of clay/polymer nanocomposites. Appl. Clay Sci. 2019, 169, 48–66. [Google Scholar] [CrossRef]
- Khanlari, S.; Kokabi, M. Thermal stability, aging properties, and flame resistance of NR-based nanocomposite. J. Appl. Polym. Sci. 2011, 119, 855–862. [Google Scholar] [CrossRef]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, P.-C.; Siddiqui, N.A.; Marom, G.; Kim, J.-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Soldano, C. Hybrid metal-based carbon nanotubes: Novel platform for multifunctional applications. Prog. Mater. Sci. 2015, 69, 183–212. [Google Scholar] [CrossRef]
- Kausar, A.; Rafique, I.; Muhammad, B. Review of applications of polymer/carbon nanotubes and epoxy/cnt composites. Polym. Plast. Technol. Eng. 2016, 55, 1167–1191. [Google Scholar] [CrossRef]
- Lau, K.T.; Hui, D. The revolutionary creation of new advanced materials—Carbon nanotube composites. Compos. Part B Eng. 2002, 33, 263–277. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, X.; Zheng, J. Study the mechanism that carbon nanotubes improve thermal stability of polymer composites: An ingenious design idea with coating silica on CNTs and valuable in engineering applications. Compos. Sci. Technol. 2018, 167, 529–538. [Google Scholar] [CrossRef]
- Bai, L.; Wang, X.; Tan, J.; Li, H.; Zheng, J. Study of distinctions in the synergistic effects between carbon nanotubes and different metal oxide nanoparticles on enhancing thermal oxidative stability of silicone rubber. J. Mater. Sci. 2016, 51, 7130–7144. [Google Scholar] [CrossRef]
- Cho, B.H.; Hwang, I.R.; Lee, Y.-S.; Jeong, J.M.; Son, K.J.; Nah, C. Enhancement of flame retardancy of rubber matrix using nanofillers. J. Nanosci. Nanotechnol. 2008, 8, 5516–5520. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.-C.; Deng, C.; Huang, S.-C.; Wei, Y.-X.; Wang, Y.-Z. Nickel-schiff base decorated graphene for simultaneously enhancing the electroconductivity, fire resistance, and mechanical properties of a polyurethane elastomer. J. Mater. Chem. A 2018, 6, 8643–8654. [Google Scholar] [CrossRef]
- Gu, J.; Liang, C.; Zhao, X.; Gan, B.; Qiu, H.; Guo, Y.; Yang, X.; Zhang, Q.; Wang, D.Y. Highly thermally conductive flame-retardant epoxy nanocomposites with reduced ignitability and excellent electrical conductivities. Compos. Sci. Technol. 2017, 139, 83–89. [Google Scholar] [CrossRef]
- Bao, C.; Guo, Y.; Song, L.; Kan, Y.; Qian, X.; Hu, Y. In situ preparation of functionalized graphene oxide/epoxy nanocomposites with effective reinforcements. J. Mater. Chem. 2011, 21, 13290–13298. [Google Scholar] [CrossRef]
- Yue, X.; Li, C.; Ni, Y.; Xu, Y.; Wang, J. Flame retardant nanocomposites based on 2D layered nanomaterials: A review. J. Mater. Sci. 2019, 54, 13070–13105. [Google Scholar] [CrossRef]
- Nine, M.J.; Tran, D.N.H.; Tran Thanh, T.; Kabiri, S.; Losic, D. Graphene-borate as an efficient fire retardant for cellulosic materials with multiple and synergetic modes of action. ACS Appl. Mater. Interfaces 2017, 9, 10160–10168. [Google Scholar] [CrossRef]
- Utracki, L.A. Clay-Containing Polymeric Nanocomposites; iSmithers Rapra Publishing: Shrewsbury, UK, 2004. [Google Scholar]
- Mittal, V.; Kim, J.K.; Pal, K. Recent Advances in Elastomeric Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent advance in research on halloysite nanotubes-polymer nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Szpilska, K.; Czaja, K.; Kudla, S. Thermal stability and flammability of polyolefin/halloysite nanotubes composites. Polimery 2015, 60, 673–679. [Google Scholar] [CrossRef]
- Sun, J.; Gu, X.; Coquelle, M.; Bourbigot, S.; Duquesne, S.; Casetta, M.; Zhang, S. Effects of melamine polyphosphate and halloysite nanotubes on the flammability and thermal behavior of polyamide 6. Polym. Adv. Technol. 2014, 25, 1552–1559. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Shi, X.; Liang, J.; Jia, Z.; Shi, G. Synergistic effect of halloysite nanotubes on flame resistance of intumescent flame retardant poly(butylene succinate) composites. Polym. Compos. 2019, 40, 202–209. [Google Scholar] [CrossRef]
- Sun, W.; Tang, W.; Gu, X.; Zhang, S.; Sun, J.; Li, H.; Liu, X. Synergistic effect of kaolinite/halloysite on the flammability and thermostability of polypropylene. J. Appl. Polym. Sci. 2018, 135, 46507. [Google Scholar] [CrossRef]
- Smith, R.J.; Holder, K.M.; Ruiz, S.; Hahn, W.; Song, Y.; Lvov, Y.M.; Grunlan, J.C. Environmentally benign halloysite nanotube multilayer assembly significantly reduces polyurethane flammability. Adv. Funct. Mater. 2018, 28, 1703289. [Google Scholar] [CrossRef]
- Boonkongkaew, M.; Sirisinha, K. Halloysite nanotubes loaded with liquid organophosphate for enhanced flame retardancy and mechanical properties of polyamide 6. J. Mater. Sci. 2018, 53, 10181–10193. [Google Scholar] [CrossRef]
- Vahabi, H.; Saeb, M.R.; Formela, K.; Cuesta, J.-M.L. Flame retardant epoxy/halloysite nanotubes nanocomposite coatings: Exploring low-concentration threshold for flammability compared to expandable graphite as superior fire retardant. Prog. Org. Coat. 2018, 119, 8–14. [Google Scholar] [CrossRef]
- Tan, W.L.; Salehabadi, A.; Isa, M.H.M.; Abu Bakar, M.; Abu Bakar, N.H.H. Synthesis and physicochemical characterization of organomodified halloysite/epoxidized natural rubber nanocomposites: A potential flame-resistant adhesive. J. Mater. Sci. 2016, 51, 1121–1132. [Google Scholar] [CrossRef]
- Yah, W.O.; Xu, H.; Soejima, H.; Ma, W.; Lvov, Y.; Takahara, A. Biomimetic dopamine derivative for selective polymer modification of halloysite nanotube lumen. J. Am. Chem. Soc. 2012, 134, 12134–12137. [Google Scholar] [CrossRef]
















| Evaluation Standards | Fire Classification | ||
|---|---|---|---|
| V-0 | V-1 | V-2 | |
| Number of samples | 5 | 5 | 5 |
| Burning time (s) | 2 | 2 | 2 |
| The flame burning time (s) of each sample after leaving fire is no more than | 10 | 30 | 30 |
| The flame burning time (s) of each group of 5 samples during 10 times of ignition after leaving fire is no more than | 50 | 250 | 250 |
| The flameless burning time (s) of each sample after the second ignition and leaving fire is no more than | 30 | 60 | 60 |
| The phenomenon that each sample has flame or flameless combustion spread to the fixture | NO | NO | NO |
| Each sample dripping ignited cotton wool | NO | NO | Yes |
| Name | UltraCarb | Hydroe Magnesite | Magnesium Hydroxide | Huntite | Aluminum Hydroxide |
|---|---|---|---|---|---|
| Chemical formula | hydromagnesite: huntite (approx. 60:40) | Mg5(CO3)4 (OH)2·4H2O | Mg(OH)2 | Mg3Ca(CO3)4 | Al(OH)3 |
| H b (J·g−1) | 990 | 1300 | 1450 | 980 | 1300 |
| Tonset (°C) | 220–240 | 220–240 | 300–320 | ~400 | 18,200 |
| Relative contribution fire retardant effects (%) | |||||
| Filler | 14 | 10 | 19 | 20 | 9 |
| Endotherm | 57 | 56 | 56 | 58 | 55 |
| Gas | 18 | 21 | 15 | 13 | 23 |
| Residue | 12 | 14 | 9 | 9 | 13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, L.; Deng, C.; Zhao, Z.-Y.; Chen, H.; Wang, Y.-Z. Flame Retardation of Natural Rubber: Strategy and Recent Progress. Polymers 2020, 12, 429. https://doi.org/10.3390/polym12020429
Wan L, Deng C, Zhao Z-Y, Chen H, Wang Y-Z. Flame Retardation of Natural Rubber: Strategy and Recent Progress. Polymers. 2020; 12(2):429. https://doi.org/10.3390/polym12020429
Chicago/Turabian StyleWan, Le, Cong Deng, Ze-Yong Zhao, Hong Chen, and Yu-Zhong Wang. 2020. "Flame Retardation of Natural Rubber: Strategy and Recent Progress" Polymers 12, no. 2: 429. https://doi.org/10.3390/polym12020429
APA StyleWan, L., Deng, C., Zhao, Z. -Y., Chen, H., & Wang, Y. -Z. (2020). Flame Retardation of Natural Rubber: Strategy and Recent Progress. Polymers, 12(2), 429. https://doi.org/10.3390/polym12020429