Efficient Removal of Pb(II) from Aqueous Solutions by Using Oil Palm Bio-Waste/MWCNTs Reinforced PVA Hydrogel Composites: Kinetic, Isotherm and Thermodynamic Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Regents
2.2. Pretreatment of OPB Fiber
2.3. Preparation of OPB/MWCNTs Reinforced PVA Hydrogel
2.4. Characterizations of OPB/MWCNTs Reinforced PVA Hydrogel
2.5. Mechanical Properties of OPB/MWCNTs Reinforced PVA Hydrogel
2.6. Batch Adsorption of Pb(II) from an Aqueous Suspension
3. Results and Discussion
3.1. Morphology Analysis
3.2. BET Surface Area Analysis
3.3. Thermal Analysis
3.4. DSC Analysis
3.5. FTIR Analysis
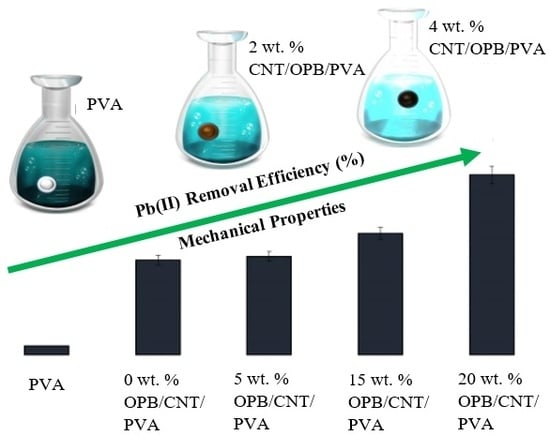
3.6. Mechanical Properties
3.6.1. Effect of Multiwalled Carbon Nanotubes (MWCNTs)
3.6.2. Effect of Oil Palm Bio-Waste (OPB)
3.7. Batch Adsorption Studies
3.7.1. Effect of Oil Palm Bio-Waste (OPB)
3.7.2. Effect of Solution pH
3.7.3. Effect of Contact Time
3.7.4. Effect of Pb(II) Concentration
3.7.5. Adsorption Kinetic Studies
3.7.6. Adsorption Isotherm Studies
3.7.7. Effect of Temperature
3.7.8. Adsorption Thermodynamics and Activation Energy
3.7.9. Comparison of OPB/PVA/MWCNTs with Other Adsorbents
4. Proposed Adsorption Mechanism of Pb(II) onto OPB/MWCNTs Reinforced PVA Hydrogel
5. Regeneration of OPB/MWCNTs Reinforced PVA Hydrogel
6. Limitations and Shortcomings
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Noor, N.M.; Othman, R.; Mubarak, N.; Abdullah, E.C. Agricultural biomass-derived magnetic adsorbents: Preparation and application for heavy metals removal. J. Taiwan Inst. Chem. Eng. 2017, 78, 168–177. [Google Scholar] [CrossRef]
- Abdelhadi, S.O.; Dosoretz, C.G.; Rytwo, G.; Gerchman, Y.; Azaizeh, H. Production of biochar from olive mill solid waste for heavy metal removal. Bioresour. Technol. 2017, 244, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Lapo, B.; Demey, H.; Carchi, T.; Sastre, A.M. Antimony removal from water by a chitosan-Iron (III)[ChiFer (III)] biocomposite. Polymers 2019, 11, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farghali, A.; Bahgat, M.; Allah, A.E.; Khedr, M. Adsorption of Pb (II) ions from aqueous solutions using copper oxide nanostructures. Beni Suef Univ. J. Basic Appl. Sci. 2013, 2, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Chipasa, K.B. Accumulation and fate of selected heavy metals in a biological wastewater treatment system. Waste Manag. 2003, 23, 135–143. [Google Scholar] [CrossRef]
- Bratskaya, S.Y.; Pestov, A.; Yatluk, Y.G.; Avramenko, V. Heavy metals removal by flocculation/precipitation using N-(2-carboxyethyl) chitosans. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 140–144. [Google Scholar] [CrossRef]
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef]
- Patterson, J.W.; Minear, R. Physical-chemical methods of heavy metals removal. In Heavy Metals in the Aquatic Environment; Elsevier: Amsterdam, The Netherlands, 2013; pp. 261–276. [Google Scholar]
- Kurniawan, T.A.; Chan, G.Y.; Lo, W.-H.; Babel, S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lim, K.-H.; Joo, K.-H.; Lee, M.-J.; Kil, S.-G.; Cho, S.-Y. Removal of heavy metal-cyanide complexes by ion exchange. Korean J. Chem. Eng. 2002, 19, 1078–1084. [Google Scholar] [CrossRef]
- Chuah, J.Y.; Chong, K.C.; Lai, S.O.; Lau, W.J.; Lee, S.S.; Ong, H.M. Industrial Nickel Wastewater Rejection by Polyimide Membrane. Chem. Eng. Trans. 2018, 63, 697–702. [Google Scholar]
- Feng, N.; Guo, X.; Liang, S.; Zhu, Y.; Liu, J. Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J. Hazard. Mater. 2011, 185, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Attar, K.; Demey, H.; Bouazza, D.; Sastre, A.M. Sorption and desorption studies of Pb (II) and Ni (II) from aqueous solutions by a new composite based on alginate and magadiite materials. Polymers 2019, 11, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Huang, R.; Peng, S.; Ma, Z. MWCNTs/cellulose hydrogels prepared from NaOH/urea aqueous solution with improved mechanical properties. J. Chem. 2015, 2015, 8. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Park, J.-S.; Park, J.-W.; Ruckenstein, E. Thermal and dynamic mechanical analysis of PVA/MC blend hydrogels. Polymers 2001, 42, 4271–4280. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Bao, S.; Wu, D.; Wang, Q.; Su, T. Functional elastic hydrogel as recyclable membrane for the adsorption and degradation of methylene blue. PLoS ONE 2014, 9, e88802. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.J.; Rabat, N.E.; Osman, N. Synthesis and characterization of oil palm empty fruit bunch-grafted-polyvinyl alcohol (OPEFB-g-PVA) hydrogel for removal of copper ions from aqueous solution. AIP Conf. Proc. 2017, 1901, 030003. [Google Scholar]
- Wang, D.; Cheng, W.; Yue, Y.; Xuan, L.; Ni, X.; Han, G. Electrospun Cellulose Nanocrystals/Chitosan/Polyvinyl Alcohol Nanofibrous Films and their Exploration to Metal Ions Adsorption. Polymers 2018, 10, 1046. [Google Scholar] [CrossRef] [Green Version]
- Lapo, B.; Demey, H.; Zapata, J.; Romero, C.; Sastre, A. Sorption of Hg (II) and Pb (II) ions on chitosan-iron (III) from aqueous solutions: Single and binary systems. Polymers 2018, 10, 367. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Bai, R. Mechanisms of lead adsorption on chitosan/PVA hydrogel beads. Langmuir 2002, 18, 9765–9770. [Google Scholar] [CrossRef]
- Ngah, W.W.; Endud, C.; Mayanar, R. Removal of copper (II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React. Funct. Polym. 2002, 50, 181–190. [Google Scholar] [CrossRef]
- Demey, H.; Barron-Zambrano, J.; Mhadhbi, T.; Miloudi, H.; Yang, Z.; Ruiz, M.; Sastre, A.M. Boron removal from aqueous solutions by using a novel alginate-based sorbent: Comparison with Al2O3 particles. Polymers 2019, 11, 1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihaela Predescu, A.; Matei, E.; Râpă, M.; Pantilimon, C.; Coman, G.; Savin, S.; Elisabeta Popa, E.; Predescu, C. Adsorption of Lead (II) from Aqueous Solution Using Chitosan and Polyvinyl Alcohol Blends. Anal. Lett. 2019, 1–28. [Google Scholar] [CrossRef]
- Karim, M.R.; Aijaz, M.O.; Alharth, N.H.; Alharbi, H.F.; Al-Mubaddel, F.S.; Awual, M.R. Composite nanofibers membranes of poly (vinyl alcohol)/chitosan for selective lead (II) and cadmium (II) ions removal from wastewater. Ecotoxicol. Environ. Saf. 2019, 169, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Afifi, A.M. Novel chitosan/polyvinyl alcohol/talc composite for adsorption of heavy metals and dyes from aqueous solution. Sep. Sci. Technol. 2018, 53, 2527–2535. [Google Scholar] [CrossRef]
- Zarghami, S.; Tofighy, M.A.; Mohammadi, T. Adsorption of zinc and lead ions from aqueous solutions using chitosan/polyvinyl alcohol membrane incorporated via acid-functionalized carbon nanotubes. J. Dispers. Sci. Technol. 2015, 36, 1793–1798. [Google Scholar] [CrossRef]
- Vatanpour, V.; Salehi, E.; Sahebjamee, N.; Ashrafi, M. Novel chitosan/polyvinyl alcohol thin membrane adsorbents modified with detonation nanodiamonds: Preparation, characterization, and adsorption performance. Arab. J. Chem. 2018, 13, 1731–1740. [Google Scholar] [CrossRef]
- Lv, L.; Chen, N.; Feng, C.; Gao, Y.; Li, M. Xanthate-modified magnetic chitosan/poly (vinyl alcohol) adsorbent: Preparation, characterization, and performance of Pb (II) removal from aqueous solution. J. Taiwan Inst. Chem. Eng. 2017, 78, 485–492. [Google Scholar] [CrossRef]
- Ajitha, P.; Vijayalakshmi, K.; Saranya, M.; Gomathi, T.; Rani, K.; Sudha, P.N.; Sukumaran, A. Removal of toxic heavy metal lead (II) using chitosan oligosaccharide-graft-maleic anhydride/polyvinyl alcohol/silk fibroin composite. Int. J. Biol. Macromol. 2017, 104, 1469–1482. [Google Scholar]
- Mallakpour, S.; Motirasoul, F. Cross-linked poly (vinyl alcohol)/modified α-manganese dioxide composite as an innovative adsorbent for lead (II) ions. J. Clean. Prod. 2019, 224, 592–602. [Google Scholar] [CrossRef]
- Abraham, T.N.; Kumar, R.; Misra, R.; Jain, S. Poly(vinyl alcohol)-based MWCNT hydrogel for lead ion removal from contaminated water. J. Appl. Polym. Sci. 2012, 125, E670–E674. [Google Scholar] [CrossRef]
- Le, V.T.; Pham, T.M.; Doan, V.D.; Lebedeva, O.E.; Nguyen, H.T. Removal of Pb (ii) ions from aqueous solution using a novel composite adsorbent of Fe3o4/PVA/spent coffee grounds. Sep. Sci. Technol. 2019, 54, 3070–3081. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, G.; Ye, L. Preparation and adsorption mechanism of polyvinyl alcohol/graphene oxide-sodium alginate nanocomposite hydrogel with high Pb (II) adsorption capacity. J. Appl. Polym. Sci. 2019, 136, 47318. [Google Scholar] [CrossRef]
- Dai, H.; Ou, S.; Liu, Z.; Huang, H. Pineapple peel carboxymethyl cellulose/polyvinyl alcohol/mesoporous silica SBA-15 hydrogel composites for papain immobilization. Carbohydr. Polym. 2017, 169, 504–514. [Google Scholar] [CrossRef]
- Sui, K.; Li, Y.; Liu, R.; Zhang, Y.; Zhao, X.; Liang, H.; Xia, Y. Biocomposite fiber of calcium alginate/multi-walled carbon nanotubes with enhanced adsorption properties for ionic dyes. Carbohydr. Polym. 2012, 90, 399–406. [Google Scholar] [CrossRef]
- Malikov, E.Y.; Muradov, M.B.; Akperov, O.H.; Eyvazova, G.M.; Puskás, R.; Madarász, D.; Nagy, L.; Kukovecz, Á.; Kónya, Z. Synthesis and characterization of polyvinyl alcohol based multiwalled carbon nanotube nanocomposites. Phys. E Low Dimens. Syst. Nanostruct. 2014, 61, 129–134. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhao, Y.M.; Hu, W.B.; Ahmad, I.; Zhu, Y.Q.; Peng, X.J.; Luan, Z.K. Carbon nanotubes-the promising adsorbent in wastewater treatment. J. Phys. Conf. Ser. 2007, 61, 698. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Ye, Z. Preparation of macroporous bead adsorbents based on poly (vinyl alcohol)/chitosan and their adsorption properties for heavy metals from aqueous solution. Chem. Eng. J. 2011, 178, 60–68. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, G.; Ye, L. In situ crosslinking of poly (vinyl alcohol)/graphene oxide Nano-composite hydrogel: Intercalation structure and adsorption mechanism for advanced Pb (II) removal. J. Polym. Res. 2018, 25, 168. [Google Scholar] [CrossRef]
- Demey, H.; Vincent, T.; Guibal, E. A novel algal-based sorbent for heavy metal removal. Chem. Eng. J. 2018, 332, 582–595. [Google Scholar] [CrossRef]
- Demey, H.; Melkior, T.; Chatroux, A.; Attar, K.; Thiery, S.; Miller, H.; Grateau, M.; Sastre, A.M.; Marchand, M. Evaluation of torrefied poplar-biomass as a low-cost sorbent for lead and terbium removal from aqueous solutions and energy co-generation. Chem. Eng. J. 2019, 361, 839–852. [Google Scholar] [CrossRef]
- Kuang, S.-P.; Wang, Z.-Z.; Liu, J.; Wu, Z.-C. Preparation of triethylene-tetramine grafted magnetic chitosan for adsorption of Pb (II) ion from aqueous solutions. J. Hazard. Mater. 2013, 260, 210–219. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, L.; Zhang, G.; Yan, T.; Yan, L.; Wei, Q.; Du, B. Removal of Pb (II) and methylene blue from aqueous solution by magnetic hydroxyapatite-immobilized oxidized multi-walled carbon nanotubes. J. Colloid Interface Sci. 2017, 494, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Ching, Y.C.; Rahman, A.; Ching, K.Y.; Sukiman, N.L.; Cheng, H.C. Preparation and characterization of polyvinyl alcohol-based composite reinforced with nanocellulose and nanosilica. Bioresources 2015, 10, 3364–3377. [Google Scholar] [CrossRef]
- Owi, W.T.; Lin, O.H.; Sam, S.T.; Chia, C.H.; Zakaria, S.; Mohaiyiddin, M.S.; Santos, G.N.; Akil, H.M. Comparative study of microcelluloses isolated from two different biomasses with commercial cellulose. Bioresources 2016, 11, 3453–3465. [Google Scholar] [CrossRef] [Green Version]
- Khoerunnisa, F.; Hendrawan; Sonjaya, Y.; Putri, O.D. Superabsorbent hydrogel composite based on copolymer cellulose/poly (vinyl alcohol)/CNT. AIP Conf. Proc. 2016, 1729, 020046. [Google Scholar]
- Zheng, T.; Liang, Y.; Ye, S.; He, Z. Superabsorbent hydrogels as carriers for the controlled-release of urea: Experiments and a mathematical model describing the release rate. Biosyst. Eng. 2009, 102, 44–50. [Google Scholar] [CrossRef]
- Zailuddin, N.L.I.; Husseinsyah, S. Tensile properties and morphology of oil palm empty fruit bunch regenerated cellulose biocomposite films. Procedia Chem. 2016, 19, 366–372. [Google Scholar] [CrossRef] [Green Version]
- Jillani, S.M.S.; Sajid, M.; Alhooshani, K. Evaluation of carbon foam as an adsorbent in stir-bar supported micro-solid-phase extraction coupled with gas chromatography–mass spectrometry for the determination of polyaromatic hydrocarbons in wastewater samples. Microchem. J. 2019, 144, 361–368. [Google Scholar] [CrossRef]
- Anitha, K.; Namsani, S.; Singh, J.K. Removal of heavy metal ions using a functionalized single-walled carbon nanotube: A molecular dynamics study. J. Phys. Chem. A 2015, 119, 8349–8358. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; He, J.; Luo, G. An improved synthesis of chitosan bead for Pb (II) adsorption. Chem. Eng. J. 2013, 226, 271–278. [Google Scholar]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A Review on Geopolymers as Emerging Materials for the Adsorption of Heavy Metals and Dyes. J. Environ. Manag. 2018, 224, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, F.; Norouzbeigi, R.; Sarbisheh, F.; Shayesteh, H. Malachite green removal using modified sphagnum peat moss as a low-cost biosorbent: Kinetic, equilibrium and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2016, 58, 482–489. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mandal, P.C.; Zulfiqar, M.; Subbarao, D. Development of ionothermal synthesis of titania nanomaterial for waste-water treatment. In Advanced Materials Research; Trans Tech Publications: Warwick, NY, USA, 2016; pp. 537–541. [Google Scholar]
- Zulfiqar, M.; Chowdhury, S.; Omar, A. Hydrothermal synthesis of multiwalled TiO2 nanotubes and its photocatalytic activities for Orange II removal. Sep. Sci. Technol. 2018, 53, 1412–1422. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Chowdhury, S.; Sufian, S.; Omar, A.A. Enhanced photocatalytic activity of orange II in aqueous solution using solvent-based TiO2 nanotubes: Kinetic, equilibrium and thermodynamic studies. J. Clean. Prod. 2018, 203, 848–859. [Google Scholar] [CrossRef]
- Demey, H.; Lapo, B.; Ruiz, M.; Fortuny, A.; Marchand, M.; Sastre, A. Neodymium recovery by chitosan/iron (III) hydroxide [ChiFer (III)] sorbent material: Batch and column systems. Polymers 2018, 10, 204. [Google Scholar] [CrossRef] [Green Version]
- Zulfiqar, M.; Samsudin, M.F.R.; Sufian, S. Modelling and optimization of photocatalytic degradation of phenol via TiO2 nanoparticles: An insight into response surface methodology and artificial neural network. J. Photochem. Photobiol. A Chem. 2019, 384, 112039. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Low, A. Fly ash based geopolymer for the adsorption of anionic surfactant from aqueous solution. J. Clean. Prod. 2019, 229, 232–243. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.; Bai, H.; Zhang, T.; Ibarra-Galvan, V.; Song, S. Methylene blue removal from water using the hydrogel beads of poly (vinyl alcohol)-sodium alginate-chitosan-montmorillonite. Carbohydr. Polym. 2018, 198, 518–528. [Google Scholar] [CrossRef]
- Zou, W.; Han, R.; Chen, Z.; Jinghua, Z.; Shi, J. Kinetic study of adsorption of Cu (II) and Pb (II) from aqueous solutions using manganese oxide coated zeolite in batch mode. Colloids Surf. A Physicochem. Eng. Asp. 2006, 279, 238–246. [Google Scholar] [CrossRef]
- Unuabonah, E.; Adebowale, K.; Olu-Owolabi, B. Kinetic and thermodynamic studies of the adsorption of lead (II) ions onto phosphate-modified kaolinite clay. J. Hazard. Mater. 2007, 144, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, G.R.; Soleymani, M.; Sabzi, M.; Azimi, H.; Atlasi, Z. Novel magnetic polyvinyl alcohol/laponite RD nanocomposite hydrogels for efficient removal of methylene blue. J. Environ. Chem. Eng. 2017, 5, 2617–2630. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanocomposite of carbon nanotubes/silica nanoparticles and their use for adsorption of Pb (II): From surface properties to sorption mechanism. Desalin. Water Treat. 2016, 57, 10730–10744. [Google Scholar] [CrossRef]
- Sekar, M.; Sakthi, V.; Rengaraj, S. Kinetics and equilibrium adsorption study of lead (II) onto activated carbon prepared from coconut shell. J. Colloid Interface Sci. 2004, 279, 307–313. [Google Scholar] [CrossRef]
- Chakravarty, S.; Mohanty, A.; Sudha, T.N.; Upadhyay, A.K.; Konar, J.; Sircar, J.K.; Madhukar, A.; Gupta, K.K. Removal of Pb (II) ions from aqueous solution by adsorption using bael leaves (Aegle marmelos). J. Hazard. Mater. 2010, 173, 502–509. [Google Scholar] [CrossRef]
- Zeytuncu, B.; Akman, S.; Yucel, O.; Kahraman, M.V. Synthesis and adsorption application of in situ photo-cross-linked electrospun poly (vinyl alcohol)-based nanofiber membranes. Water Air Soil Pollut. 2015, 226, 173. [Google Scholar] [CrossRef]
- Kul, A.R.; Koyuncu, H. Adsorption of Pb (II) ions from aqueous solution by native and activated bentonite: Kinetic, equilibrium and thermodynamic study. J. Hazard. Mater. 2010, 179, 332–339. [Google Scholar] [CrossRef]
- Li, Q.; Chai, L.; Yang, Z.; Wang, Q. Kinetics and thermodynamics of Pb (II) adsorption onto modified spent grain from aqueous solutions. Appl. Surf. Sci. 2009, 255, 4298–4303. [Google Scholar] [CrossRef]
- Futalan, C.M.; Kim, J.; Yee, J.-J. Adsorptive treatment via simultaneous removal of copper, lead and zinc from soil washing wastewater using spent coffee grounds. Water Sci. Technol. 2019, 79, 1029–1041. [Google Scholar] [CrossRef]
- Din, M.I.; Hussain, Z.; Mirza, M.L.; Shah, A.T.; Athar, M.M. Adsorption optimization of lead (II) using Saccharum bengalense as a non-conventional low cost biosorbent: Isotherm and thermodynamics modeling. Int. J. Phytoremed. 2014, 16, 889–908. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; Huang, H. Eco-friendly polyvinyl alcohol/carboxymethyl cellulose hydrogels reinforced with graphene oxide and bentonite for enhanced adsorption of methylene blue. Carbohydr. Polym. 2018, 185, 1–11. [Google Scholar] [CrossRef]
















| Hydrogel Composite | Solutions | Adsorption Capacity | Ref. |
|---|---|---|---|
| Chitosan/PVA beads | Chitosan/PVA mixed ratio = 75:25–25:75, glutaraldehyde = 5% (v/v), acetic acid = 1% (wt %), deionized water = 552 g | 9.48 mg/g | [25] |
| Chitosan/PVA blend nanofiber membrane | Chitosan, distilled water, PVA, acetic acid = 2% (wt %) | 266.12 mg/g | [26] |
| Cellulose/chitosan/PVA nanofibrous films | Microcrystalline cellulose = 40 g, water = 4.7 g, H2SO4 (98 wt %) = 65.3 g, chitosan:acetic acid = 90:10 (vol %), chitosan:PVA = 60:40 (vol %) | 323.49 mg/g | [20] |
| Chitosan/PVA beads | Chitosan flaks = 4.26 g, dilute acetic acid = 2% (w/w), PVA = 8.51 g, deionized water = 100 mL | - | [22] |
| Chitosan/PVA thin membrane | Chitosan powder, acetic acid = 1% (v/v), PVA sol. = 10 wt %, nanodiamonds = 0–1.5 wt % | 121.3 mg/g | [29] |
| Chitosan/PVA talc composite | PVA = 8 wt %, Chitosan = 7 wt %, distilled water, chitosan:acetic acid (concentrated) = 50:50, talc = 1 wt % | 88% | [27] |
| Chitosan/PVA | PVA = appropriate amount, sodium alginate = 1.3 g, CaCO3 powder, distilled water = 150 mL, chitosan = certain amount, CaCl2-saturated boric acid sol. = 3% | 166.44 mg/g | [40] |
| Chitosan/MWCNTs/PVA hydrogel membrane | Chitosan sol. = 2 wt %, acetic acid sol. = 2 wt %, PVA sol. = 2 wt % | - | [28] |
| Xanthate-modified with Fe3O4-based chitosan/PVA hydrogel | Chitosan = 6 g, aqueous acetic acid = 150 mL (2% v/v), PVA = 6 g, deionized water = 150 mL, Fe3O4 = 6 g, | 97.8% | [30] |
| Fe3O4/PVA/spent coffee ground | Spent coffee ground = 100 mesh screen, FeCl3/Na2SO3 = mixed sol., Fe3O4 particles, PVA sol. = 2 wt % (w/v), spent coffee ground:Fe3O4 = 1:1–6:1 (wt %) | 0.275 mmol/g | [34] |
| Chitosan oligosaccharide-g-maleic anhydride/PVA/ silk fibroin composite | Silk fibroin = 0.25 g, 0.5 wt % Na2CO3, ceric ammonium nitrate = 0.5 g, 1 N HNO3 = 10 mL, PVA = 1 mL, chitosan oligosaccharide = 5 g, cocoons, maleic anhydride = 2.5 g, distilled water = 30 mL | 16.412 mg/g | [31] |
| PVA/α-manganese dioxide composite | MnSO4.H2O = 200 mg, KMnO4 = 500 mg, deionized water = 10 mL, ethanol = 9 mL, PVA = 300 mg, H2SO4 = 1 mL | 88.7% | [32] |
| Graphene oxide/PVA nano-composite hydrogel | Graphene oxide = 0.5 g, deionized water = 100 mL, ethylenediamine-triacetic acid sodium = appropriate amount | 67% | [41] |
| PVA/MWCNTs | MWCNTs = 0.2 g, PVA solution = 500 mL, glutaraldehyde = 10 mL (2.5 %), HCl = 1% | 86% | [33] |
| PVA/graphene oxide-sodium alginate nanocomposite hydrogel | Graphene oxide = 1–5 g, deionized water = 100 mL, sodium alginate = 5 g, PVA = 1–5 g, mixed solution of boric acid and CaCl2 | 279.43 mg/g | [35] |
| Algal-based sorbent | Polyethyleneimine (PEI) = 6 g, water = 200 mL, glutaraldehyde (50% w/w) = 6 mL. Alginate/PEI beads: sodium alginate (4% w/w) = 50 g, PEI derivative powder = 2 g, calcium carbonate sol. (10% w/w) = 2 g, water = 46 g. Alginate/Fucus/PEI beads: Fucus vesiculosus alga = 10 g, sodium carbonate = 2 g, water = 288 g, PEI derivative powder = 2 g, sodium alginate = 1 g, calcium carbonate (10 % w/w) = 2 g, water = 35 g | 1.09 mmol/g | [42] |
| Torrefied biomass | CENTORRE oven = i.d: ϕ 1.82 m, hearth height: 0.74 m including rotatory axis i.d: ϕ 0.42 m, flow rate of biomass ≥ 12 kg/hr, torrefaction conditions = 250 and 280 °C for 75 and 60 min, respectively | 30.0 mg/g | [43] |
| Oil palm bio-waste/MWCNTs/PVA composite hydrogel | PVA = 26 g, distilled water = 200 mL, OPB = 5–20 wt %, MWCNTs = 1–4 wt %, NMBA = 0.08 g, APS = 1.25 g, washed with acetone | 30.031 mg/g | This study |
| Hydrogels | Accessible Porosity (%) | Total Surface Area (m2/g) | Pore Diameter (nm) |
|---|---|---|---|
| PVA | 0.07 | 12.49 | 8.45 |
| MWCNTs/PVA | 2.816 | 31.11 | |
| 5 wt % OPB/4 wt % MWCNTs/PVA | 2.861 | 31.54 | |
| 15 wt % OPB/4 wt % MWCNTs/PVA | 2.352 | 39.75 | |
| 30 wt % OPB/4 wt % MWCNTs/PVA | 7.53 | 3.382 | 110.98 |
| Adsorbents | Experimental | PFO Kinetic Model | PSO Kinetic Model | |||||
|---|---|---|---|---|---|---|---|---|
| Conc. (ppm) | Qe, exp. (mg/g) | Qe, cal. (mg/g) | K1 × 10−3 (min−1) | R2 | Qe, cal. (mg/g) | K1 × 10−4 (min−1) | R2 | |
| PVA | 65 | 11.121 | 11.714 | −1.401 | 0.974 | 11.401 | 6.981 | 0.898 |
| 150 | 33.175 | 34.969 | −2.511 | 0.922 | 33.101 | 4.012 | 0.868 | |
| 200 | 41.221 | 41.554 | −2.812 | 0.928 | 41.011 | 7.161 | 0.964 | |
| OPB/PVA | 65 | 1.626 | 1.091 | −10.821 | 0.942 | 1.591 | 1252.5 | 0.991 |
| 150 | 32.938 | 32.616 | −5.182 | 0.928 | 32.735 | 1.441 | 0.989 | |
| 200 | 78.912 | 73.886 | −3.591 | 0.977 | 78.627 | 1.372 | 0.996 | |
| OPB/PVA/MWCNTs | 65 | 1.672 | 1.778 | −13.587 | 0.951 | 1.763 | 45.101 | 0.998 |
| 150 | 15.817 | 14.067 | −9.991 | 0.957 | 15.542 | 7.641 | 0.962 | |
| 200 | 35.148 | 25.763 | −8.752 | 0.916 | 35.214 | 3.572 | 0.942 | |
| Isotherms | Parameters | PVA | OPB/PVA | OPB/PVA/MWCNTs |
|---|---|---|---|---|
| Langmuir | qm (mg/g) | 18.329 | 13.021 | 30.031 |
| KL (L/mg) | 3.534 | 0.791 | 0.779 | |
| R2 | 0.973 | 0.968 | 0.989 | |
| Freundlich | KF (mg/g) | −0.449 | 1.263 | 1.013 |
| n | 1.141 | 5.772 | 4.291 | |
| R2 | 0.966 | 0.922 | 0.987 | |
| Temkin | KT (L/g) | 0.539 | 1.451 | 1.876 |
| B | 53.476 | 7.514 | 7.821 | |
| R2 | 0.944 | 0.925 | 0.915 |
| T (K) | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (kJ/mol K) | Ea (kJ/mol) |
|---|---|---|---|---|
| 298 | −16.461 | 28.904 | 55.193 | 12.551 |
| 308 | −17.012 | |||
| 318 | −17.563 |
| Type of Adsorbents | Temperature Range (K) | Activation Energy (kJ/mol) | Ref. |
|---|---|---|---|
| Activated carbon | 305–315 | 35.528 | [67] |
| Bael leaves | 303–323 | 22.20 | [68] |
| PVA-based nanofiber membrane | 298–318 | 20.29 | [69] |
| Native bentonite | 303–328 | 16.51 | [70] |
| Activated bentonite | 303–328 | 15.62 | [70] |
| MWCNTs/silica nanocomposite | 295–335 | 15.80 | [66] |
| Spent grain | 288–318 | 12.33 | [71] |
| Manganese oxide coated zeolite | 288–328 | 11.90 | [63] |
| Spent coffee ground | 288–328 | 11.84 | [72] |
| Unmodified kaolinite clay | 298–323 | 11.90, 19.0, 5.12 | [64] |
| Phosphate modified kaolinite clay | 298–323 | 5.64, 10.68, 4.32 | [64] |
| Saccharum bengalense | 283–333 | 5.054 | [73] |
| OPB/PVA/MWCNTs hydrogel | 298–318 | 12.551 | Present study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulfiqar, M.; Lee, S.Y.; Mafize, A.A.; Kahar, N.A.M.A.; Johari, K.; Rabat, N.E. Efficient Removal of Pb(II) from Aqueous Solutions by Using Oil Palm Bio-Waste/MWCNTs Reinforced PVA Hydrogel Composites: Kinetic, Isotherm and Thermodynamic Modeling. Polymers 2020, 12, 430. https://doi.org/10.3390/polym12020430
Zulfiqar M, Lee SY, Mafize AA, Kahar NAMA, Johari K, Rabat NE. Efficient Removal of Pb(II) from Aqueous Solutions by Using Oil Palm Bio-Waste/MWCNTs Reinforced PVA Hydrogel Composites: Kinetic, Isotherm and Thermodynamic Modeling. Polymers. 2020; 12(2):430. https://doi.org/10.3390/polym12020430
Chicago/Turabian StyleZulfiqar, Muhammad, San Yi Lee, Amira Azreena Mafize, Nur Adlin Mastura Abdul Kahar, Khairiraihanna Johari, and Nurul Ekmi Rabat. 2020. "Efficient Removal of Pb(II) from Aqueous Solutions by Using Oil Palm Bio-Waste/MWCNTs Reinforced PVA Hydrogel Composites: Kinetic, Isotherm and Thermodynamic Modeling" Polymers 12, no. 2: 430. https://doi.org/10.3390/polym12020430
APA StyleZulfiqar, M., Lee, S. Y., Mafize, A. A., Kahar, N. A. M. A., Johari, K., & Rabat, N. E. (2020). Efficient Removal of Pb(II) from Aqueous Solutions by Using Oil Palm Bio-Waste/MWCNTs Reinforced PVA Hydrogel Composites: Kinetic, Isotherm and Thermodynamic Modeling. Polymers, 12(2), 430. https://doi.org/10.3390/polym12020430