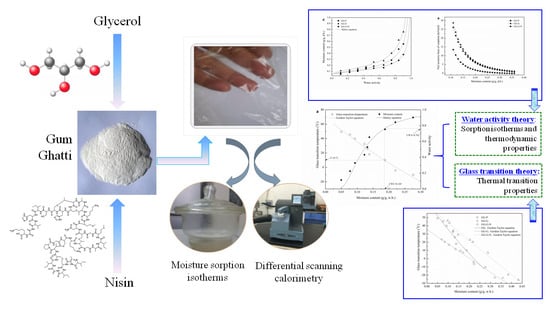
Thermodynamic Properties and State Diagram of Gum Ghatti-Based Edible Films: Effects of Glycerol and Nisin
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of Films
2.3. Moisture Sorption Isotherms
2.4. Thermodynamic Properties
2.4.1. Net Isosteric Heat of Sorption (qst) and Differential Entropy (Sd)
2.4.2. Enthalpy-Entropy Compensation Theory
2.4.3. Spreading Pressure (φ)
2.5. Glass Transition Temperature (Tg)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Moisture Sorption Isotherm
3.2. Thermodynamic Properties
3.2.1. Net Isosteric Heat of Sorption and Differential Entropy
3.2.2. Enthalpy-entropy Compensation Theory
3.2.3. Spreading Pressure
3.3. Glass Transition Temperature (Tg)
3.4. State Diagram of GG-Based Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arcan, I.; Yemenicioğlu, A. Incorporating phenolic compounds opens a new perspective to use zein films as flexible bioactive packaging materials. Food Res. Int. 2011, 44, 550–556. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Zhao, Y.; Warner, R.D.; Stuart, J. Active and intelligent packaging in meat industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Nieto, M.B. Structure and Function of Polysaccharide Gum-Based Edible Films and Coating. In Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 57–112. [Google Scholar]
- Zhang, P.; Zhao, Y.; Shi, Q. Characterization of a novel edible film based on gum ghatti: Effect of plasticizer type and concentration. Carbohydr. Polym. 2016, 153, 345–355. [Google Scholar] [CrossRef]
- Fabra, M.J.; Talens, P.; Chiralt, A. Water sorption isotherms and phase transitions of sodium caseinate–lipid films as affected by lipid interactions. Food Hydrocoll. 2010, 24, 384–391. [Google Scholar] [CrossRef]
- Rhim, J.W.; Lee, J.H. Thermodynamic analysis of water vapor sorption isotherms and mechanical properties of selected paper-based food packaging materials. J. Food Sci. 2009, 74, E502–E511. [Google Scholar] [CrossRef]
- Xiao, Q.; Tong, Q. Thermodynamic properties of moisture sorption in pullulan–sodium alginate based edible films. Food Res. Int. 2013, 54, 1605–1612. [Google Scholar] [CrossRef]
- Al-Muhtaseb, A.H.; McMinn, W.A.M.; Magee, T.R.A. Moisture sorption isotherm characteristics of food products: A review. Food Bioprod. Process 2002, 80, 118–128. [Google Scholar] [CrossRef]
- McMinn, W.A.M.; Magee, T.R.A. Thermodynamic properties of moisture sorption of potato. J. Food Eng. 2003, 60, 157–165. [Google Scholar] [CrossRef]
- Bonilla, E.; Azuara, E.; Beristain, C.I.; Vernon-Carter, E.J. Predicting suitable storage conditions for spray-dried microcapsules formed with different biopolymer matrices. Food Hydrocoll. 2010, 24, 633–640. [Google Scholar] [CrossRef]
- Biliaderis, C.G.; Lazaridou, A.; Arvanitoyannis, I. Glass transition and physical properties of polyol-plasticised pullulan–starch blends at low moisture. Carbohydr. Polym. 1999, 40, 29–47. [Google Scholar] [CrossRef]
- Sablani, S.S.; Syamaladevi, R.M.; Swanson, B.G. A review of methods, data and applications of state diagram of food systems. Food Eng. Rev. 2010, 2, 168–203. [Google Scholar] [CrossRef]
- Shi, Q.; Lin, W.; Zhao, Y.; Zhang, P. Thermal characteristics and state diagram of Penaeus vannamei meat with and without maltodextrin addition. Thermochim. Acta 2015, 616, 92–99. [Google Scholar] [CrossRef]
- Arfat, Y.A. Plasticizers for Biopolymer Films. In Glass Transition and Phase Transition in Food and Biological Materials; Ahmed, J., Ed.; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Kurek, M.; Guinault, A.; Voilley, A.; Galic, K.; Debeaufort, F. Effect of relative humidity on carvacrol release and permeation properties of chitosan based films and coatings. Food Chem. 2014, 144, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remedio, L.N.; Silva dos Santos, J.W.; Vieira Maciel, V.B.; Yoshida, C.M.P.; Aparecida de Carvalho, R. Characterization of active chitosan films as a vehicle of potassium sorbate or nisin antimicrobial agents. Food Hydrocoll. 2019, 87, 830–838. [Google Scholar] [CrossRef]
- Marvdashti, L.M.; Koocheki, A.; Yavarmanesh, M. Characterization, release profile and antimicrobial properties of bioactive polyvinyl alcohol-Alyssum homolocarpum seed gum-nisin composite film. Food Biophys. 2019, 14, 120–131. [Google Scholar] [CrossRef]
- Aguirre-Loredo, R.Y.; Rodriguez-Hernandez, A.I.; Velazquez, G. Modelling the effect of temperature on the water sorption isotherms of chitosan films. Food Sci. Technol. 2016, 37, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhang, P.; Shi, Q. Effect of Nisin on the properties of edible films prepared by gum ghatti. Sci. Technol. Food Ind. 2017, 38, 69–73. [Google Scholar]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. Sect. A 1977, 81, 89–102. [Google Scholar] [CrossRef]
- McLaughlin, C.P.; Magee, T.R.A. The determination of sorption isotherm and the isosteric heats of sorption for potatoes. J. Food Eng. 1998, 35, 267–280. [Google Scholar] [CrossRef]
- Shi, Q.; Lin, W.; Zhao, Y.; Zhang, P.; Wang, R. Moisture adsorption isotherms and thermodynamic properties of Penaeus vannamei meat with and without maltodextrin addition. J. Aquat. Food Prod. Technol. 2016, 25, 1348–1367. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Torres, M.D.; Vallejo, N. Thermodynamic analysis of experimental sorption isotherms of loquat and quince fruits. J. Food Eng. 2008, 88, 514–521. [Google Scholar] [CrossRef]
- Noshad, M.; Mohebbi, M.; Shahidi, F.; Mortazavi, S.A. Effect of osmosis and ultrasound pretreatment on the moisture adsorption isotherms of quince. Food Bioprod. Process. 2012, 90, 266–274. [Google Scholar] [CrossRef]
- Lago, C.C.; Liendo-Cárdenas, M.; Noreña, C.P.Z. Thermodynamic sorption properties of potato and sweet potato flakes. Food Bioprod. Process. 2013, 91, 389–395. [Google Scholar] [CrossRef]
- Krug, R.R.; Hunter, W.G.; Grieger, R.A. Enthalpy-entropy compensation. 2. Separation of the chemical from the statistical effect. J. Phys. Chem. 1976, 80, 2341–2351. [Google Scholar] [CrossRef]
- McMinn, W.A.M.; Al-Muhtaseb, A.H.; Magee, T.R.A. Enthalpy–entropy compensation in sorption phenomena of starch materials. Food Res. Int. 2005, 38, 505–510. [Google Scholar] [CrossRef]
- Alpizar-Reyes, E.; Carrillo-Navas, H.; Romero-Romero, R.; Varela-Guerrero, V.; Alvarez-Ramírez, J.; Pérez-Alonso, C. Thermodynamic sorption properties and glass transition temperature of tamarind seed mucilage (Tamarindus indica L.). Food Bioprod. Process. 2017, 101, 166–176. [Google Scholar] [CrossRef]
- Brett, B.; Figueroa, M.; Sandoval, A.J.; Barreiro, J.A.; Müller, A.J. Moisture sorption characteristics of starchy products: Oat flour and rice flour. Food Biophys. 2009, 4, 151–157. [Google Scholar] [CrossRef]
- Toğrul, H.; Arslan, N. Moisture sorption behaviour and thermodynamic characteristics of rice stored in a chamber under controlled humidity. Biosyst. Eng. 2006, 95, 181–195. [Google Scholar] [CrossRef]
- Spada, J.C.; Noreña, C.P.Z.; Marczak, L.D.F.; Tessaro, I.C. Water adsorption isotherms of microcapsules with hydrolyzed pinhão (Araucaria angustifolia seeds) starch as wall material. J. Food Eng. 2013, 114, 64–69. [Google Scholar] [CrossRef]
- Cefalas, A.C.; Sarantopoulou, E.; Kollia, Z.; Kitsara, M.; Raptis, I.; Bakalis, E. Entropic nanothermodynamic potential from molecular trapping within photon induced nano-coids in photon processed PDMS layer. Soft Matter 2012, 8, 5561–5574. [Google Scholar] [CrossRef]
- Gavriil, V.; Chatzichristidi, M.; Kollia, Z.; Cefalas, A.C.; Spyropoulos-Antonakakis, N.; Semashko, V.V.; Sarantopoulou, E. Photos probe entropic potential variation during molecular confinement in nanocavities. Entropy 2018, 20, 545. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.D.; Moreira, R.; Chenlo, F.; Vazquez, M.J. Water adsorption isotherms of carboxymethyl cellulose, guar, locust bean, tragacanth and xanthan gums. Carbohydr. Polym. 2012, 89, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S. Food stability determination by macro–micro region concept in the state diagram and by defining a critical temperature. J. Food Eng. 2010, 99, 402–416. [Google Scholar] [CrossRef]
- Xiao, Q.; Lim, L.-T.; Tong, Q. Properties of pullulan-based blend films as affected by alginate content and relative humidity. Carbohydr. Polym. 2012, 87, 227–234. [Google Scholar] [CrossRef]





| Saturated Salt Solutions | Temperature (°C) | ||
|---|---|---|---|
| 5 | 15 | 25 | |
| LiCl | 0.113 | 0.113 | 0.113 |
| CH3COOK | 0.235 | 0.234 | 0.225 |
| MgCl2 | 0.336 | 0.333 | 0.328 |
| K2CO3 | 0.431 | 0.432 | 0.432 |
| Mg(NO3)2 | 0.589 | 0.559 | 0.529 |
| NaNO2 | 0.693 | 0.693 | 0.654 |
| NaCl | 0.757 | 0.756 | 0.753 |
| KCl | 0.877 | 0.859 | 0.843 |
| BaCl2 | 0.931 | 0.915 | 0.903 |
| Model | Parameter | Temperature (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 15 | 25 | ||||||||
| GG-P | GG-G | GG-G-N | GG-P | GG-G | GG-G-N | GG-P | GG-G | GG-G-N | ||
| Xmg (g/g) | 0.080 | 0.118 | 0.133 | 0.086 | 0.111 | 0.127 | 0.080 | 0.096 | 0.127 | |
| Cg | 20.00 | 24.23 | 23.41 | 21.67 | 16.51 | 31.91 | 19.90 | 15.62 | 13.72 | |
| GAB | K | 0.87 | 0.92 | 0.93 | 0.86 | 0.90 | 0.93 | 0.87 | 0.92 | 0.92 |
| R2 | 0.985 | 0.995 | 0.992 | 0.993 | 0.996 | 0.991 | 0.985 | 0.984 | 0.988 | |
| SE | 0.0151 | 0.0170 | 0.0258 | 0.0106 | 0.0131 | 0.0264 | 0.0246 | 0.0299 | 0.0299 | |
| Residual pattern | Scattered | Scattered | Patterned | Scattered | Scattered | |||||
| a | 0.013 | 0.0380 | 0.062 | 0.016 | 0.040 | 0.068 | 0.017 | 0.044 | 0.072 | |
| r | 2.148 | 1.830 | 1.709 | 1.942 | 1.709 | 1.587 | 1.836 | 1.537 | 1.493 | |
| Halsey | R2 | 0.989 | 0.991 | 0.988 | 0.989 | 0.989 | 0.987 | 0.985 | 0.980 | 0.984 |
| SE | 0.0134 | 0.0203 | 0.0309 | 0.0126 | 0.0203 | 0.0305 | 0.0137 | 0.0259 | 0.0320 | |
| Residual pattern | Scattered | Scattered | Scattered | |||||||
| Samples | Tβ (K) | ΔGβ (kJ/mol) | R2 |
|---|---|---|---|
| GG-P | 359.3 | 535.0 | 0.999 |
| GG-G | 332.4 | 598.6 | 0.999 |
| GG-G-N | 335.2 | 1031.6 | 0.999 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Zhao, Y.; Zhang, X.; Zhu, L.; Fang, Z.; Shi, Q. Thermodynamic Properties and State Diagram of Gum Ghatti-Based Edible Films: Effects of Glycerol and Nisin. Polymers 2020, 12, 449. https://doi.org/10.3390/polym12020449
Zhang P, Zhao Y, Zhang X, Zhu L, Fang Z, Shi Q. Thermodynamic Properties and State Diagram of Gum Ghatti-Based Edible Films: Effects of Glycerol and Nisin. Polymers. 2020; 12(2):449. https://doi.org/10.3390/polym12020449
Chicago/Turabian StyleZhang, Pingping, Ya Zhao, Xin Zhang, Lanlan Zhu, Zhongxiang Fang, and Qilong Shi. 2020. "Thermodynamic Properties and State Diagram of Gum Ghatti-Based Edible Films: Effects of Glycerol and Nisin" Polymers 12, no. 2: 449. https://doi.org/10.3390/polym12020449
APA StyleZhang, P., Zhao, Y., Zhang, X., Zhu, L., Fang, Z., & Shi, Q. (2020). Thermodynamic Properties and State Diagram of Gum Ghatti-Based Edible Films: Effects of Glycerol and Nisin. Polymers, 12(2), 449. https://doi.org/10.3390/polym12020449