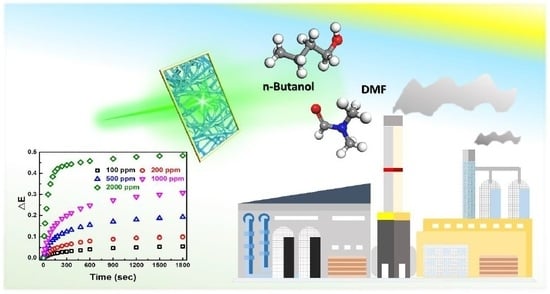
Highly-Sensitive Detection of Volatile Organic Compound Vapors by Electrospun PANI/P3TI/PMMA Fibers
Abstract
:1. Introduction
2. Experiment Section
2.1. Preparation of PANI/P3TI/PMMA Fibers
2.2. Characterization
2.3. VOC Detection
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andre, R.S.; Sanfelice, R.C.; Pavinatto, A.; Mattoso, L.H.C.; Correa, D.S. Hybrid nanomaterials designed for volatile organic compounds sensors: A review. Mater. Des. 2018, 156, 154–166. [Google Scholar] [CrossRef]
- Elosua, C.; Matias, I.R.; Bariain, C.; Arregui, F.J. Volatile organic compound optical fiber sensors: A review. Sensors 2006, 6, 1440–1465. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Sauvé, G.; Iovu, M.C.; Jeffries-El, M.; Zhang, R.; Cooper, J.; Santhanam, S.; Schultz, L.; Revelli, J.C.; Kusne, A.G.; et al. Volatile organic compound detection using nanostructured copolymers. Nano Lett. 2006, 6, 1598–1602. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-K.; Chang, G.T.; Thokchom, A.K.; Kim, T.; Park, J. Ultra-fast responsive colloidal–polymer composite-based volatile organic compounds (VOC) sensor using nanoscale easy tear process. Sci. Rep. 2018, 8, 5291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.-H.; Heo, J.-M.; Jeong, W.; Yoo, Y.H.; Park, B.J.; Kim, J.-M. Smartphone-based VOC sensor using colorimetric polydiacetylenes. ACS Appl. Mater. Interfaces 2018, 10, 5014–5021. [Google Scholar] [CrossRef]
- Hu, H.; Trejo, M.; Nicho, M.E.; Saniger, J.M.; Garcı́a-Valenzuela, A. Adsorption kinetics of optochemical NH3 gas sensing with semiconductor polyaniline films. Sens. Actuators B 2002, 82, 14–23. [Google Scholar] [CrossRef]
- Janzen, M.C.; Ponder, J.B.; Bailey, D.P.; Ingison, C.K.; Suslick, K.S. Colorimetric sensor arrays for volatile organic compounds. Anal. Chem. 2006, 78, 3591–3600. [Google Scholar] [CrossRef]
- Mahesh, K.; Karpagam, S.; Pandian, K. How to design donor–acceptor based heterocyclic conjugated polymers for applications from organic electronics to sensors. Top. Curr. Chem. 2019, 377, 12. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Chemiresistive polyaniline-based gas sensors: A mini review. Sens. Actuators B 2015, 220, 534–548. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Joo, B.-S.; Choi, N.-J.; Lim, J.-O.; Huh, J.-S.; Lee, D.-D. Visible optical sensing of ammonia based on polyaniline film. Sens. Actuators B 2003, 93, 148–152. [Google Scholar] [CrossRef]
- Nicho, M.E.; Trejo, M.; Garcı́a-Valenzuela, A.; Saniger, J.M.; Palacios, J.; Hu, H. Polyaniline composite coatings interrogated by a nulling optical-transmittance bridge for sensing low concentrations of ammonia gas. Sens. Actuators B 2001, 76, 18–24. [Google Scholar] [CrossRef]
- Sajad, P. Chemiresistive gas sensors based on conducting polymers. In Materials Science and Engineering: Concepts, Methodologies, Tools, and Applications; IGI Global: Hershey, PA, USA, 2017; pp. 543–574. [Google Scholar]
- Macagnano, A.; Perri, V.; Zampetti, E.; Bearzotti, A.; De Cesare, F. Humidity effects on a novel eco-friendly chemosensor based on electrospun PANI/PHB nanofibres. Sens. Actuators B 2016, 232, 16–27. [Google Scholar] [CrossRef]
- Sonker, R.K.; Yadav, B.C. Development of Fe2O3–PANI nanocomposite thin film based sensor for NO2 detection. J. Taiwan Inst. Chem. Eng. 2017, 77, 276–281. [Google Scholar] [CrossRef]
- Zhu, C.; Cheng, X.; Dong, X.; Xu, Y.M. Enhanced sub-ppm NH3 gas sensing performance of PANI/TiO2 nanocomposites at room temperature. Front. Chem. 2018, 6, 493. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Mishra, S.; Shimpi, N.G. Synthesis and sensing applications of polyaniline nanocomposites: A review. RSC Adv. 2016, 6, 42196–42222. [Google Scholar] [CrossRef]
- Jeevananda, T. Synthesis and characterization of polyaniline filled PU/PMMA interpenetrating polymer networks. Eur. Polym. J. 2003, 39, 569–578. [Google Scholar] [CrossRef]
- Kumar, L.; Rawal, I.; Kaur, A.; Annapoorni, S. Flexible room temperature ammonia sensor based on polyaniline. Sens. Actuators B 2017, 240, 408–416. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, E.; Kim, K.; Lee, B.H.; Choe, S. Polyaniline effect on the conductivity of the PMMA/Ag hybrid composite. Colloids Surf. A 2012, 396, 195–202. [Google Scholar] [CrossRef]
- Gibson, G.L.; McCormick, T.M.; Seferos, D.S. Atomistic band gap engineering in donor–acceptor polymers. J. Am. Chem. Soc. 2012, 134, 539–547. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Wang, J. Structures and properties of conjugated donor–acceptor copolymers for solar cell applications. J. Mater. Chem. 2012, 22, 4178–4187. [Google Scholar] [CrossRef]
- Zhu, Y.; Champion, R.D.; Jenekhe, S.A. Conjugated donor−acceptor copolymer semiconductors with large intramolecular charge transfer: Synthesis, optical properties, electrochemistry, and field effect carrier mobility of thienopyrazine-based copolymers. Macromolecules 2006, 39, 8712–8719. [Google Scholar] [CrossRef]
- Lu, C.-F.; Shih, C.-W.; Chen, C.-A.; Chin, A.; Su, W.-F. Tuning the morphology of isoindigo donor–acceptor polymer film for high sensitivity ammonia sensor. Adv. Funct. Mater. 2018, 28, 1803145. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Van Hoang, N.; Hung, C.M.; Hoa, N.D.; Van Duy, N.; Park, I.; Van Hieu, N. Excellent detection of H2S gas at ppb concentrations using ZnFe2O4 nanofibers loaded with reduced graphene oxide. Sens. Actuators B 2019, 282, 876–884. [Google Scholar] [CrossRef]
- Abutalib, M.M. Insights into the structural, optical, thermal, dielectric, and electrical properties of PMMA/PANI loaded with graphene oxide nanoparticles. Physica B 2019, 552, 19–29. [Google Scholar] [CrossRef]
- Araújo, P.L.B.; Araújo, E.S.; Santos, R.F.S.; Pacheco, A.P.L. Synthesis and morphological characterization of PMMA/polyaniline nanofiber composites. Microelectron. J. 2005, 36, 1055–1057. [Google Scholar] [CrossRef]
- Veluru, J.B.; Satheesh, K.K.; Trivedi, D.C.; Ramakrishna, M.V.; Srinivasan, N.T. Electrical properties of electrospun fibers of PANI-PMMA composites. J. Eng. Fibers Fabr. 2007, 2, 155892500700200203. [Google Scholar] [CrossRef] [Green Version]
- Vu, D.L.; Li, Y.-Y.; Lin, T.-H.; Wu, M.-C. Fabrication and humidity sensing property of UV/ozone treated PANI/PMMA electrospun fibers. J. Taiwan Inst. Chem. Eng. 2019, 99, 250–257. [Google Scholar] [CrossRef]
- Ho, C.-C.; Chen, C.-A.; Chang, C.-Y.; Darling, S.B.; Su, W.-F. Isoindigo-based copolymers for polymer solar cells with efficiency over 7%. J. Mater. Chem. A 2014, 2, 8026–8032. [Google Scholar] [CrossRef]
- Kumar, V.; Yokozeki, T.; Goto, T.; Takahashi, T. Synthesis and characterization of PANI-DBSA/DVB composite using roll-milled PANI-DBSA complex. Polymer 2016, 86, 129–137. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Tsao, C.W.; Hromada, L.; Liu, J.; Kumar, P.; DeVoe, D.L. Low temperature bonding of PMMA and COC microfluidic substrates using UV/ozone surface treatment. Lab Chip 2007, 7, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sun, R.; Xu, S.; Guo, J.; Wang, W.; Guo, J.; Jiao, X.; Wang, J.; Jia, S.; Zhu, X.; et al. A wide-bandgap D–A copolymer donor based on a chlorine substituted acceptor unit for high performance polymer solar cells. J. Mater. Chem. A 2019, 7, 14070–14078. [Google Scholar] [CrossRef]
- Wu, M.-C.; Lin, C.-H.; Lin, T.-H.; Chan, S.-H.; Chang, Y.-H.; Lin, T.-F.; Zhou, Z.; Wang, K.; Lai, C.-S. Ultrasensitive detection of volatile organic compounds by a freestanding aligned Ag/CdSe–CdS/PMMA texture with double-side UV–Ozone treatment. ACS Appl. Mater. Interfaces 2019, 11, 34454–34462. [Google Scholar] [CrossRef]
- Deng, S.; Liu, X.; Chen, N.; Deng, D.; Xiao, X.; Wang, Y. A highly sensitive VOC gas sensor using p-type mesoporous Co3O4 nanosheets prepared by a facile chemical coprecipitation method. Sens. Actuators B 2016, 233, 615–623. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, D.L.; Lin, T.-F.; Lin, T.-H.; Wu, M.-C. Highly-Sensitive Detection of Volatile Organic Compound Vapors by Electrospun PANI/P3TI/PMMA Fibers. Polymers 2020, 12, 455. https://doi.org/10.3390/polym12020455
Vu DL, Lin T-F, Lin T-H, Wu M-C. Highly-Sensitive Detection of Volatile Organic Compound Vapors by Electrospun PANI/P3TI/PMMA Fibers. Polymers. 2020; 12(2):455. https://doi.org/10.3390/polym12020455
Chicago/Turabian StyleVu, Duy Linh, Tz-Feng Lin, Ting-Han Lin, and Ming-Chung Wu. 2020. "Highly-Sensitive Detection of Volatile Organic Compound Vapors by Electrospun PANI/P3TI/PMMA Fibers" Polymers 12, no. 2: 455. https://doi.org/10.3390/polym12020455
APA StyleVu, D. L., Lin, T. -F., Lin, T. -H., & Wu, M. -C. (2020). Highly-Sensitive Detection of Volatile Organic Compound Vapors by Electrospun PANI/P3TI/PMMA Fibers. Polymers, 12(2), 455. https://doi.org/10.3390/polym12020455