Analysis of Thermal–Mechanical Properties of Silicon Dioxide/Polyvinylidene Fluoride Reinforced Non-Woven Fabric (Polypropylene) Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
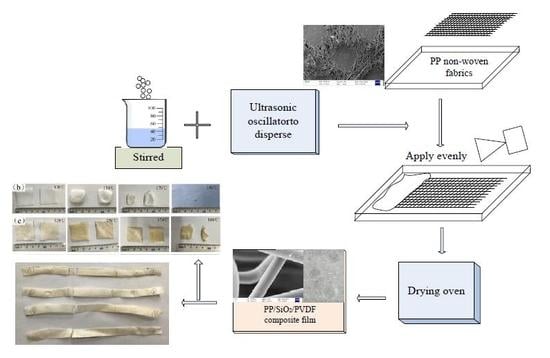
2.2. Preparation of Composite Films
2.3. Materials Characterization
3. Results
3.1. Analysis of Mechanical Properties of Composite Materials
3.1.1. Uniaxial Tensile Mechanical Properties of PP/SiO2/PVDF Composites
3.1.2. Effect of Temperature on the Mechanical Properties of PP/SiO2/PVDF Composites
3.2. Thermal Stability Analysis
3.3. Microstructure Characterization
3.3.1. SEM and EDS Analysis
3.3.2. XRD Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, W.; Song, Z.; Zhong, J.; Qian, J.; Tan, Z.; Wu, X.; Chu, H.; Nie, W.; Ran, X. Multilayer-structured transparent MXene/PVDF film with excellent dielectric and energy storage performance. J. Mater. Chem. C. 2019, 7, 10371–10378. [Google Scholar] [CrossRef]
- Tian, G.; Deng, W.; Gao, Y.; Xiong, D.; Yan, C.; He, X.; Yan, W. Rich lamellar crystal baklava-structured PZT/PVDF piezoelectric sensor toward individual table tennis training. Nano Energy 2019, 59, 574–581. [Google Scholar] [CrossRef]
- Ji, X.; Chen, D.; Zheng, Y.; Shen, J.; Guo, S.; Harkin-Jones, E. Multilayered assembly of poly(vinylidene fluoride) and poly(methyl methacrylate) for achieving multi-shape memory effects. Chem. Eng. J. 2019, 362, 190–198. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, H.; Xu, Y.; Liu, T.; Hou, T.; Liu, L.; Xin, Y. Preparation of PVDF/TiO2 nanofibers with enhanced piezoelectric properties for geophone applications. Smart Mater. Struct. 2019, 28, 085006. [Google Scholar] [CrossRef]
- Iezzi, R.A.; Gaboury, S.; Wood, K. Acrylic-fluoropolymer mixtures and their use in coatings. Prog. Org. Coat. 2000, 40, 55–60. [Google Scholar] [CrossRef]
- Szewczyk, P.K.; Metwally, S.; Krysiak, Z.J.; Kaniuk, Ł.; Karbowniczek, J.E.; Stachewicz, U. Enhanced osteoblasts adhesion and collagen formation on biomimetic polyvinylidene fluoride (PVDF) films for bone regeneration. Biomed. Mater. 2019, 14, 065006. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Tan, C.; Shi, K.; Li, J.; Wang, X.X.; Sun, B.; Jiang, P. Wireless piezoelectric devices based on electrospun PVDF/BaTiO3 NW nanocomposite fibers for human motion monitoring. Nanoscale 2018, 10, 17751–17760. [Google Scholar] [CrossRef] [PubMed]
- Dudem, B.; Kim, D.H.; Bharat, L.K.; Yu, J.S. Highly-flexible piezoelectric nanogenerators with silver nanowires and barium titanate embedded composite films for mechanical energy harvesting. Appl. Energy 2018, 230, 865–874. [Google Scholar] [CrossRef]
- Motta, E.P.; Reis, J.M.L.; Da Costa Mattos, H.S. Analysis of the cyclic tensile behaviour of an elasto-viscoplastic polyvinylidene fluoride (PVDF). Polym. Test. 2018, 67, 503–512. [Google Scholar] [CrossRef]
- Wang, Z.; Kong, F.; Chang, M.; Yun, K. A comprehensive investigation of particle effect on the mechanical property, hydrophobicity and permeability of polymer composite membranes based on a chain model. J. Membr. Sci. 2019, 590, 117302. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, Z.; Xie, A.; Meng, M.; Cui, Y.; Liu, S.; Dong, H. Bio-inspired fabrication of superhydrophilic nanocomposite membrane based on surface modification of SiO2 anchored by polydopamine towards effective oil-water emulsions separation. Sep. Purif. Technol. 2019, 209, 434–442. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, L. Preparation and characterization of novel poly (vinylidene fluoride) membranes using flower-like Bi2WO6 for membrane distillation. J. Taiwan Inst. Chem. Eng. 2017, 80, 867–874. [Google Scholar] [CrossRef]
- Damtie, M.M.; Kim, B.; Woo, Y.C.; Choi, J.S. Membrane distillation for industrial wastewater treatment: Studying the effects of membrane parameters on the wetting performance. Chemosphere 2018, 206, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Lin, H.; Yang, L.; Tong, Q.; Pan, F.; Weng, J.; Zheng, S. High thermal stability and low impedance polypropylene separator coated with aluminum phosphate. Electrochim. Acta 2019, 320, 134528. [Google Scholar] [CrossRef]
- Jeong, H.S.; Choi, E.S.; Lee, S.Y.; Kim, J.H. Evaporation-induced, close-packed silica nanoparticle-embedded nonwoven composite separator membranes for high-voltage/high-rate lithium-ion batteries: Advantageous effect of highly percolated, electrolyte-philic microporous architecture. J. Membr. Sci. 2012, 415, 513–519. [Google Scholar] [CrossRef]
- Gong, W.; Wei, S.; Ruan, S.; Shen, C. Electrospun coaxial PPESK/PVDF fibrous membranes with thermal shutdown property used for lithium-ion batteries. Mater. Lett. 2019, 244, 126–129. [Google Scholar] [CrossRef]
- Kong, F.; Chang, M.; Wang, Z. Investigation of the effect of nanosilica on mechanical, structural, and fracture toughness of polyvinylidene fluoride films. Mater. Res. Express 2019, 6, 105369. [Google Scholar] [CrossRef]
- Xu, K.; Qin, Y.; Xu, T.; Xie, X.; Deng, J.; Qi, J.; Huang, C. Combining polymeric membranes with inorganic woven fabric: Towards the continuous and affordable fabrication of a multifunctional separator for lithium-ion battery. J. Membr. Sci. 2019, 592, 117364. [Google Scholar] [CrossRef]











| Materials | Strain (-) | Stress (MPa) | Contrast of Stress (%) |
|---|---|---|---|
| PP | 1.221 | 7.493 | - |
| PP/PVDF | 0.428 | 11.661 | 155.62 |
| PP/SiO2/PVDF (2%) | 0.389 | 15.346 | 204.80 |
| PP/SiO2/PVDF (4%) | 0.294 | 18.314 | 244.42 |
| PP/SiO2/PVDF (6%) | 0.252 | 12.396 | 165.43 |
| PP/SiO2/PVDF (8%) | 0.344 | 11.901 | 158.82 |
| Materials | Stress at 50 °C (MPa) | Strain at 50 °C (-) | Stress at 80 °C (MPa) | Strain at 80 °C (-) |
|---|---|---|---|---|
| PP/PVDF | 10.183 | 0.395 | 9.394 | 0.319 |
| PP/SiO2/PVDF (2%) | 10.826 | 0.281 | 10.906 | 0.256 |
| PP/SiO2/PVDF (4%) | 12.303 | 0.298 | 12.254 | 0.239 |
| PP/SiO2/PVDF (6%) | 11.951 | 0.253 | 10.764 | 0.241 |
| PP/SiO2/PVDF (8%) | 10.102 | 0.252 | 9.070 | 0.240 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, F.; Chang, M.; Wang, Z. Analysis of Thermal–Mechanical Properties of Silicon Dioxide/Polyvinylidene Fluoride Reinforced Non-Woven Fabric (Polypropylene) Composites. Polymers 2020, 12, 481. https://doi.org/10.3390/polym12020481
Kong F, Chang M, Wang Z. Analysis of Thermal–Mechanical Properties of Silicon Dioxide/Polyvinylidene Fluoride Reinforced Non-Woven Fabric (Polypropylene) Composites. Polymers. 2020; 12(2):481. https://doi.org/10.3390/polym12020481
Chicago/Turabian StyleKong, Fangyun, Mengzhou Chang, and Zhenqing Wang. 2020. "Analysis of Thermal–Mechanical Properties of Silicon Dioxide/Polyvinylidene Fluoride Reinforced Non-Woven Fabric (Polypropylene) Composites" Polymers 12, no. 2: 481. https://doi.org/10.3390/polym12020481
APA StyleKong, F., Chang, M., & Wang, Z. (2020). Analysis of Thermal–Mechanical Properties of Silicon Dioxide/Polyvinylidene Fluoride Reinforced Non-Woven Fabric (Polypropylene) Composites. Polymers, 12(2), 481. https://doi.org/10.3390/polym12020481