Improvement of Mechanical and Self-Healing Properties for Polymethacrylate Derivatives Containing Maleimide Modified Graphene Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Maleimide Modified GO (mGO)
2.3. Preparation of Furan Functionalized Polymethacrylate
2.4. Preparation of Self-Healable Furan-Functionalized Polymethacrylate Nanocomposite Films
2.5. Characterization Methods
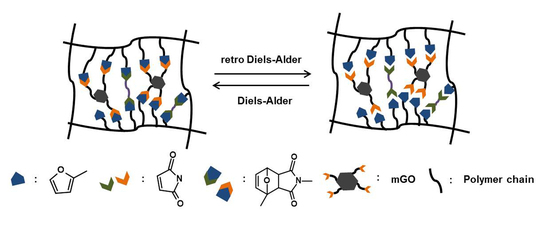
3. Results
3.1. Synthesis and Characterization of mGO
3.2. Preparation of FEEMA# Copolymers
3.3. Characterization of FEEMA64 Nanocomposites
3.4. Mechanical Properties of FEEMA64 Nanocomposite
3.5. Self-Healing Property
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tee, B.; Wang, C.; Allen, R.; Bao, Z. An electrically and mechanically self-healing composite with pressure- and flexion-sensitive properties for electronic skin application. Nat. Nanotechnol. 2012, 7, 825–832. [Google Scholar] [CrossRef]
- Zhu, D.; Lu, X.; Lu, Q. Electrically Conductive PEDOT Coating with Self-Healing Superhydrophobicity. Langmuir 2014, 30, 4671–4677. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jiang, B.; Huang, Y. Self-Healable polysiloxane/graphene nanocomposite and its application in pressure sensor. J. Mater. Sci. 2019, 54, 5472–5483. [Google Scholar] [CrossRef]
- Li, J.; Liang, J.; Li, L.; Ren, F.; Hu, W.; Li, J.; Qi, S.; Pei, Q. Healable Capacitive Touch Screen Sensors Based on Transparent Composite Electrode Comprising Silver Nanowires and a Furan/Maleimide Diels-Alder Cycloaddition Polymer. ACS Nano 2014, 8, 12–12882. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. A tough, smart elastomeric bio-based hyperbranched polyurethane nanocomposite. New J. Chem. 2015, 39, 2146–2154. [Google Scholar] [CrossRef]
- Ling, L.; Li, J.; Zhang, G.; Sun, R.; Wong, C.P. Self-Healing and Shape Memory Linear Polyurethane Based on Disulfide Linkages with Excellent Mechanical Property. Macromol. Res. 2018, 26, 365–373. [Google Scholar] [CrossRef]
- Zhang, W.; Zhan, Y.; Gao, X.; Li, R.; Zhu, W.; Xu, H.; Liu, B.; Fang, X.; Xu, Y.; Ding, T. Effect of oxygen functionalities of graphene oxide on polymerization and thermal properties of reactive benzoxazine nanocomposite. Macromol. Res. 2018, 26, 77–84. [Google Scholar] [CrossRef]
- Zeng, J.; Li, J.; Yuan, P.; Zhang, P. Theoretical Prediction of Heat Transport in Few-Layer Graphene/Epoxy Composites. Macromol. Res. 2018, 26, 978–983. [Google Scholar] [CrossRef]
- Peterson, A.M.; Jensen, R.E.; Palmese, G.R. Thermoreversible and remendable glass-polymer interface for fiber-reinforced composite. Compos. Sci. Technol. 2011, 72, 568–592. [Google Scholar] [CrossRef] [Green Version]
- Yim, Y.J.; Bae, K.M.; Park, S.J. Influence of Oxyfluorination on Geometrical Pull-Out Behavior of Carbon-Fiber-Reinforced Epoxy Matrix Composites. Macromol. Res. 2018, 26, 794. [Google Scholar] [CrossRef]
- Jin, F.L.; Zhang, H.; Yao, S.S.; Park, S.J. Effect of Surface Modification on Impact Strength and Flexural Strength of Poly(lactic acid)/Silicon Carbide Nanocomposite. Macromol. Res. 2018, 26, 211–214. [Google Scholar] [CrossRef]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Canadell, J.; Goossens, H.; Klumperman, B. Self-healing materials based on disulfide links. Macromolecules 2011, 44, 2536–2541. [Google Scholar] [CrossRef]
- Yoon, J.A.; Kamada, J.; Koynov, J.; Mohin, J.; Nicolay, R.; Zhang, Y.; Balazs, A.C.; Kosalewaski, T.; Matyjaszewski, K. Self-healing polymer films based on thiol-disulfide exchange reactions and self-healing kinetics measured using atomic force microscopy. Macromolecules 2012, 45, 142–149. [Google Scholar] [CrossRef]
- Rao, Y.L.; Chortos, A.; Pfattner, R.; Lissel, F.; Chiu, Y.C.; Feig, V.; Xu, J.; Kurosawa, T.; Gu, X.; Wang, C.; et al. Stretchable Self-Healing Polymeric Dielectrics Cross-Linked Through Metal-Ligand Coordination. J. Am. Chem. Soc. 2016, 138, 6020–6027. [Google Scholar] [CrossRef]
- Cui, J.; del Champo, A. Multivalent H-bonds for self-healing hydrogels. Chem. Commun. 2012, 48, 9302–9304. [Google Scholar] [CrossRef]
- Klukovich, H.M.; Kean, Z.S.; lacono, S.T.; Craig, S.L. Mechanically Induced Scission and Subsequent Thermal Remending of Perfluorocyclobutane Polymers. J. Am. Chem. Soc. 2011, 133, 17882–17888. [Google Scholar] [CrossRef]
- Chung, C.-M.; Roh, Y.-S.; Cho, S.-Y.; Kim, J.-G. Crack Healing in Polymeric Materials via Photochemical [2+2] Cycloaddition. Chem. Mater. 2004, 16, 3982–3984. [Google Scholar] [CrossRef]
- Froimowicz, P.; Frey, H.; Landfester, K. Towards the Generation of Self-Healing Materials by Means of a Reversible Photo-induced Approach. Macromol. Rapid Commun. 2011, 32, 468–473. [Google Scholar] [CrossRef]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A Thermally Re-mendable Cross-Linked Polymeric Material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Zhou, L.-L.; Lang, S.-Y.; Lu, H.-Y.; Wang, D.; Chen, C.-F.; Wan, L.-J. Click and patterned functionalization of graphene by Diels-Alder reaction. J. Am. Chem. Soc. 2016, 138, 7448–7451. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, G.; Tatsi, E.; Rigatelli, B.; Turri, S.; Griffini, G. Highly Transparent and Colorless Self-Healing Polyacrylate Coatings Based on Diels-Alder Chemistry. Macromol. Mater. Eng. 2020, 305, 1900652. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Yuan, L.; Liang, G.; Gu, A. Simultaneously achieving high strength, thermal resistance and high self-healing efficiency for polyacrylate coating by constructing a Diels-Alder reversible covalent structure with multi-maleimide terminated hyperbranched polysiloxane. Polym. Int. 2020, 69, 110–120. [Google Scholar] [CrossRef]
- Kavitha, A.A.; Singha, N.K. “Click Chemistry” in Tailor-Made Polymethacrylates Bearing Reactive Furfuryl Functionality: A New Class of Self-Healing Polymeric Material. ACS Appl. Mater. Interfaces 2009, 1, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Hanaique, J.; Gogoi, J.; Nath, J.; Kumar Dolui, S. Synthesis of Self-Healing Bio-Based Tannic Acid-Based Methacrylates By Thermoreversible Diels-Alder Reaction. Polym. Eng. Sci. 2020, 60, 140–150. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, J.; Lyu, B.; Ma, J.; Yang, Z. Polyacrylate crosslinked with furyl alcohol grafting bismaleimide: A self-healing polymer coating. Prog. Org. Coat. 2020, 139, 105475. [Google Scholar] [CrossRef]
- Yang, S.; Du, X.; Du, Z.; Zhou, M.; Cheng, X.; Wang, H.; Yan, B. Robust, stretchable and photothermal self-healing polyurethane elastomer based on furan-modified polydopamine nanoparticles. Polymer 2020, 190, 122219. [Google Scholar] [CrossRef]
- Lima, M.R.; Orozco, F.; Picchioni, F.; Moreno-Villoslada, I.; Pucci, A.; K. Bose, R.; Araya-Hermosilla, R. Electrically Self-Healing Thermoset MWCNTs Composites Based on Diels-Alder and Hydrogen Bonds. Polymers 2019, 11, 1885. [Google Scholar] [CrossRef] [Green Version]
- Tanasi, P.; Santa, M.H.; Carretero-Gonzálz, J.; Verdejo, R.; López-Manchado, M.A. Thermo-reversible crosslinked natural rubber: A Diels-Alder route for reuse and self-healing properties in elastomers. Polymer 2019, 175, 15–24. [Google Scholar] [CrossRef]
- Luan, Y.G.; Zhang, X.A.; Jiang, S.L.; Chen, J.H.; Lyu, Y.F. Self-healing Supramolecular Polymer Composites by Hydrogen Bonding Interactions between Hyperbranched Polymer and Graphene Oxide. Chin. J. Polym. Sci. 2018, 36, 584–591. [Google Scholar] [CrossRef]
- Mao, J.; Zhao, C.; Li, Y.; Xiang, D.; Wang, Z. Highly stretchable, self-healing, and strain-sensitive based on double-crosslinked nanocomposite hydrogel. Compos. Commun. 2020, 17, 22–27. [Google Scholar] [CrossRef]
- Utera-Barrios, S.; Hernández Santana, M.; Verdejo, R.; López-Manchado, M.A. Design of Rubber Composites with Autonomous Self-Healing Capability. ACS Omega 2020, 5, 1902–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Elia, E.; Barg, S.; Ni, N.; Rocha, V.G.; Saiz, E. Self-Healing Graphene-Based Composites with Sensing Capabilities. Adv. Mater. 2015, 27, 4788–4794. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, N.; Allen, R.; Tok, J.B.H.; Wu, Y.; Zhang, F.; Chen, Y.; Bao, Z. A Rapid and Efficient Self-Healing Thermo-Reversible Elastomer Crosslinked with Graphene Oxide. Adv. Mater. 2013, 25, 5785–5790. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Meng, Y.; Li, Y. Electric heating behavior of flexible graphene/natural rubber conductor with self-healing conductive network. Mater. Lett. 2017, 192, 115–118. [Google Scholar] [CrossRef]
- Huang, L.; Yi, N.; Wu, Y.; Zhang, Y.; Zhang, Q.; Huang, Y.; Ma, Y.; Chen, Y. Multichannel and Repeatable Self-Healing of Mechanical Enhanced Graphene-Thermoplastic Polyurethane Composites. Adv. Mater. 2013, 25, 2224–2228. [Google Scholar] [CrossRef]
- Hernández, M.; Bernal, M.M.; Grande, A.M.; Zhong, N.; Zwaag, S.; García, S.J. Effect of graphene content on the restoration of mechanical, electrical and thermal functionalities of a self-healing natural rubber. Smart Mater. Struct. 2017, 26, 085010. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offerman, R.E.J. Preparation of Graphitic oxide. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Cha, S.-H. Enhancement of self-healing property by introducing ethylene glycol group into thermally reversible diels-alder reaction based self-healable materials. Macromol. Res. 2017, 25, 640–647. [Google Scholar] [CrossRef]
- Raghubanshi, H.; Ngobeni, S.M.; Osikoya, A.O.; Shooto, N.D.; Dikio, C.W.; Naidoo, E.B.; Dikio, E.D.; Pandey, R.K.; Prakash, R. Synthesis of graphene oxide and its application for the adsorption of Pb+2 from aqueous solution. J. Ind. Eng. 2017, 47, 169–178. [Google Scholar] [CrossRef]
- Sainsbury, T.; Gnaniah, S.; Spencer, S.J.; Mignuzzi, S.; Belsey, N.A.; Paton, K.R.; Satti, A. Extreme mechanical reinforcement in graphene oxide based thin-film nanocomposites via covalently tailored nanofiller matrix compatibilization. Carbon 2017, 114, 367–376. [Google Scholar] [CrossRef]
- Kim, J.T.; Kim, B.K.; Kim, E.Y.; Kwon, S.H.; Jeong, H.M. Synthesis and properties of near IR induced self-healable polyurethane/graphene nanocomposites. Eur. Polym. J. 2013, 49, 3889–3896. [Google Scholar] [CrossRef]
- Chen, C.; Yang, Q.-H.; Yang, Y.; Lv, W.; Wen, Y.; Hou, P.-X.; Wang, M.; Cheng, H.-M. Self-Assembled Free-Standing Graphite Oxide Membrane. Adv. Mater. 2009, 21, 3007–3011. [Google Scholar]
- McAllister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single Sheet Functionalized Graphene by Oxidation and Thermal Expansion of Graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Yang, A.; Li, J.; Zhang, C.; Zhang, W.; Ma, N. One-step amine modification of graphene oxide to get a green trifunctional metal-free catalyst. Appl. Surf. Sci. 2015, 346, 443–450. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J. Graphene/Polyaniline Nanofiber Composites as Supercapacitor Electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Pokharel, P.; Lee, D.S. High performance polyurethane nanocomposite films prepared from a masterbatch of graphene oxide in polyether polyol. Chem. Eng. J. 2014, 253, 356–365. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Teng, C.-C.; Ma, C.-C.M.; Lu, C.-H.; Yang, S.-Y.; Lee, S.-H.; Hsiao, M.-C.; Yen, M.-Y.; Chiou, K.-C.; Lee, T.-M. Thermal conductivity and structure of non-covalent funcationlized graphene/epoxy composites. Carbon 2011, 49, 5107–5116. [Google Scholar] [CrossRef]
- Nasr, F.H.; Barikani, M.; Salehirad, M. Preparation of self-healing polyurethane/functionalized graphene nanocomposites as electro-conductive one part adhesives. RSC Adv. 2018, 8, 31094–31105. [Google Scholar] [CrossRef] [Green Version]
- Byun, K.-S.; Choi, W.J.; Lee, H.-Y.; Sim, M.-J.; Cha, S.-H.; Lee, J.-C. The effect of electron density in furan pendant group on thermal-reversible Diels-Alder reaction based self-healing properties of polymethacrylate derivatives. RSC Adv. 2018, 8, 39432–39443. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.I.; Halder, S.; Wang, J. Diels-Alder based epoxy matrix and interfacial healing of bismaleimide grafted GNP infused hybrid nanocomposites. Polym. Test. 2019, 74, 138–151. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Sun, R.; Wong, C.-P. A covalently cross-linked reduced functionalized graphene oxide/polyurethane composite based on Diels-Alder chemistry and its potential application in healable flexible electronics. J. Mater Chem. C 2017, 5, 220–288. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/Polymer Nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Zhuang, Y.-N.; Wang, H.-T.; Wei, M.-F.; Ko, W.-C.; Chang, W.-J.; Way, T.-F.; Rwei, S.-P. Fabrication of Self-Healable Magnetic Nanocomposites via Diels−Alder Click Chemistry. Appl. Sci. 2019, 9, 506. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.; She, X.; Yang, D.; Wu, X.; Su, F.; Chen, Y. Synthesis of network reduced graphene oxide in polystyrene matrix by a two-step reduction method for superior conductivity of the composite. J. Mater. Chem. 2012, 22, 17254–17261. [Google Scholar] [CrossRef]
- Lin, C.; Sheng, D.; Liu, X.; Xu, S.; Ji, F.; Dong, L.; Zhou, Y.; Yang, Y. NIR induced self-healing electrical conductivity polyurethane/graphene nanocomposites based on Diels-Alder reaction. Polymer 2018, 140, 150–157. [Google Scholar] [CrossRef]
- Bhawal, P.; Ganguly, S.; Chaki, T.K.; Das, N.C. Synthesis and characterization of graphene oxide filled ethylene methyl acylate hybrid nanocomposites. RSC Adv. 2016, 6, 20781–20790. [Google Scholar] [CrossRef]
- Lin, C.; Sheng, D.; Liu, X.; Xu, S.; Ji, F.; Dong, L.; Zhou, Y.; Yang, Y. A self-healable nanocomposite based on dual-crosslinked Graphene Oxide/Polyurethane. Polymer 2017, 127, 241–250. [Google Scholar] [CrossRef]










| C (Atomic %) | H (Atomic %) | N (Atomic %) | O (Atomic %) | |
|---|---|---|---|---|
| Theoretical value | 39.286 | 42.857 | - | 17.857 |
| Experimental value | 35.484 | 48.387 | - | 16.129 |
| Sample | Before Healing Test | After Healing Test | Self-Healing Efficiency 1 (%) | ||
|---|---|---|---|---|---|
| Tensile Strength (MPa) | Elongation at Break (%) | Tensile Strength (MPa) | Elongation at Break (%) | ||
| FEEMA64 polymer film | 2.648 ± 0.257 | 214.667 ± 14.687 | 1.663 ± 0.261 | 12.600 ± 0.898 | 5.724 |
| FEEMA64_mGO0.015 wt% | 4.021 ± 0.229 | 198.235 ± 10.232 | 3.611 ± 0.155 | 36.465 ± 12.070 | 23.560 |
| FEEMA64_mGO0.030 wt% | 4.566 ± 0.564 | 69.035 ± 9.383 | 2.916 ± 0.171 | 61.465 ± 1.322 | 81.228 |
| FEEMA64_mGO0.050 wt% | 4.022 ± 0.542 | 155.510 ± 6.980 | 2.927 ± 0.151 | 141.333 ± 7.588 | 82.033 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-J.; Cha, S.-H. Improvement of Mechanical and Self-Healing Properties for Polymethacrylate Derivatives Containing Maleimide Modified Graphene Oxide. Polymers 2020, 12, 603. https://doi.org/10.3390/polym12030603
Lee W-J, Cha S-H. Improvement of Mechanical and Self-Healing Properties for Polymethacrylate Derivatives Containing Maleimide Modified Graphene Oxide. Polymers. 2020; 12(3):603. https://doi.org/10.3390/polym12030603
Chicago/Turabian StyleLee, Won-Ji, and Sang-Ho Cha. 2020. "Improvement of Mechanical and Self-Healing Properties for Polymethacrylate Derivatives Containing Maleimide Modified Graphene Oxide" Polymers 12, no. 3: 603. https://doi.org/10.3390/polym12030603
APA StyleLee, W. -J., & Cha, S. -H. (2020). Improvement of Mechanical and Self-Healing Properties for Polymethacrylate Derivatives Containing Maleimide Modified Graphene Oxide. Polymers, 12(3), 603. https://doi.org/10.3390/polym12030603