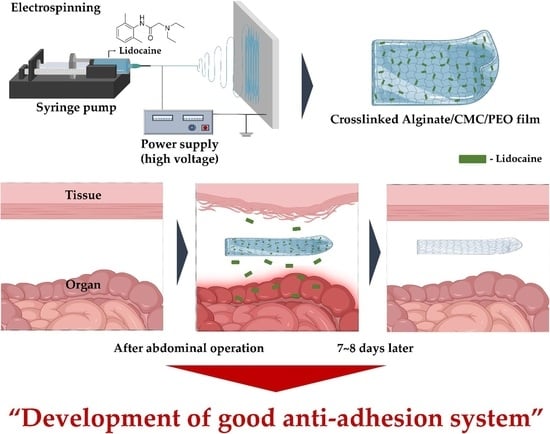
Development of a Lidocaine-Loaded Alginate/CMC/PEO Electrospun Nanofiber Film and Application as an Anti-Adhesion Barrier
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Preparation of the Alginate/CMC/PEO Solution for Electrospinning
2.3. Fabrication of Electrospun Films via Electrospinning
2.4. Crosslinking of Electrospun Films Using a CaCl2 Solution
2.5. Cell Culture
2.6. Evaluation of Cell Experiments on Alginate/CMC/PEO Nanofiber Films
2.7. Characterization of the Alginate/CMC/PEO Nanofiber Films
2.8. Preparation of HPLC Samples and Lidocaine Release Test
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Alginate/CMC/PEO Nanofiber Films
3.2. Analysis of the Alginate/CMC/PEO Nanofiber Film Using FT-IR
3.3. In Vitro Experiment with Alginate/CMC/PEO Nanofiber Films
3.4. Evaluation of Lidocaine Release Behavior by Crosslinking Degree of Nanofibrous Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ellis, H.J. The causes and prevention of intestinal adhesions. Br. J. Surg. 1982, 69, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Liakakos, T.; Thomakos, N.; Fine, P.M.; Dervenis, C.; Young, R.L. Peritoneal adhesions: Etiology, pathophysiology, and clinical significance. Dig. Surg. 2001, 18, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.C.; Panitch, A. Abdominal adhesions: Current and novel therapies. J. Surg. Res. 2011, 165, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Alponat, A.; Lakshminarasappa, S.R.; Yavuz, N.; Goh, P.M. Prevention of adhesions by Seprafilm, an absorbable adhesion barrier: An incisional hernia model in rats. Am. Surg. 1997, 63, 818–819. [Google Scholar]
- Mettler, L.; Audebert, A.; Lehmann-Willenbrock, E.; Schive, K.; Jacobs, V.R. Prospective clinical trial of SprayGel as a barrier to adhesion formation: An interim analysis. J. Am. Assoc. Gynecol. Laparosc. 2003, 10, 339–344. [Google Scholar] [CrossRef]
- Kim, D.; Choi, G.; Seungho, B.; Abdullah, A.; Jang, S.; Hong, S.A.; Kim, B.; Lee, J.; Kang, H.; Lee, D. Characterization of Anti-Adhesion Properties of Alginate/Polyethylene Oxide Film to Reduce Postsurgical Peritoneal Adhesions. Sci. Adv. Mater. 2017, 9, 1669–1677. [Google Scholar] [CrossRef]
- Chkhikvadze, T.F.; Zarnadze, N.K. Soluble synthetic suture materials. Khirurgiia 1990, 12, 154–158. [Google Scholar]
- Perrin, B.R.M.; Dupeux, M.; Tozzi, P.; Delay, D.; Gersbach, P.; von Segesser, L.K. Surgical glues: Are they really adhesive? Eur. J. Cardio Thorac. Surg. 2009, 36, 967–972. [Google Scholar] [CrossRef]
- Bakkum, E.A.; Dalmeijer, R.A.; Verdel, M.J.; Hermans, J.; van Blitterswijk, C.A.; Trimbos, J.B. Quantitative analysis of the inflammatory reaction surrounding sutures commonly used in operative procedures and the relation to postsurgical adhesion formation. Biomaterials 1995, 16, 1283–1289. [Google Scholar] [CrossRef]
- Marianowski, L.; Barcz, E. Biologic tissue response to sutures. Ginekol. Pol. 2004, 75, 570–577. [Google Scholar]
- Cui, X.; Abdullah, A.; Lee, J.; Kang, H.; Lee, D. Controlled Release of Antibiotics from Air Jet Spun Polycaprolactone Scaffolds. Sci. Adv. Mater. 2017, 9, 1533–1539. [Google Scholar] [CrossRef]
- Baek, S.; Park, H.; Kim, M.; Lee, D. Preparation of PCL/(+)-catechin/gelatin film for wound healing using air-jet spinning. Appl. Surf. Sci. 2019, 145033. [Google Scholar] [CrossRef]
- Wannaphatchaiyong, S.; Heng, P.W.S.; Suksaeree, J.; Boonme, P.; Pichayakorn, W. Lidocaine loaded gelatin/gelatinized tapioca starch films for buccal delivery and the irritancy evaluation using chick chorioallantoic membrane. Saudi Pharm. J. 2019, 27, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, Y.; Li, Z. Topical anesthesia therapy using lidocaine-loaded nanostructured lipid carriers: Tocopheryl polyethylene glycol 1000 succinate-modified transdermal delivery system. Drug Des. Dev. 2018, 12, 4231–4240. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.M.; Ullah, A.; Chang, C.H.; Kim, G.M. Preparation of lidocaine-loaded porous Poly (lactic-co-glycolic acid) microparticles using microfluidic flow focusing and phosphate buffer solution porogen. Int. J. Precis. Eng. Manuf. 2017, 18, 599–604. [Google Scholar] [CrossRef]
- Görner, T.; Gref, R.; Michenot, D.; Sommer, F.; Tran, M.N.; Dellacherie, E. Lidocaine-loaded biodegradable nanospheres. I. Optimization of the drug incorporation into the polymer matrix. J. Control. Release 1999, 57, 259–268. [Google Scholar] [CrossRef]
- Loughlin, R.G.; Tunney, M.M.; Donnelly, R.F.; Murphy, D.J.; Jenkins, M.; McCarron, P.A. Modulation of gel formation and drug-release characteristics of lidocaine-loaded poly(vinyl alcohol)-tetraborate hydrogel systems using scavenger polyol sugars. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2008, 69, 1135–1146. [Google Scholar] [CrossRef]
- Anton, F. Process and apparatus for preparing artificial threads. Google Patents : 2, October, 1934. [Google Scholar]
- Gupta, K.C.; Haider, A.; Choi, Y.-R.; Kang, I.-K. Nanofibrous scaffolds in biomedical applications. Biomater. Res. 2014, 18, 5. [Google Scholar] [CrossRef] [Green Version]
- Uppal, R.; Ramaswamy, G.N.; Arnold, C.; Goodband, R.; Wang, Y. Hyaluronic acid nanofiber wound dressing—Production, characterization, and in vivo behavior. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 20–29. [Google Scholar] [CrossRef]
- Wu, X.-M.; Branford-White, C.J.; Zhu, L.-M.; Chatterton, N.P.; Yu, D.-G. Ester prodrug-loaded electrospun cellulose acetate fiber mats as transdermal drug delivery systems. J. Mater. Sci. Mater. Med. 2010, 21, 2403–2411. [Google Scholar] [CrossRef]
- Tungprapa, S.; Jangchud, I.; Supaphol, P.J. Release characteristics of four model drugs from drug-loaded electrospun cellulose acetate fiber mats. Polymer 2007, 48, 5030–5041. [Google Scholar] [CrossRef]
- Zeng, J.; Xu, X.; Chen, X.; Liang, Q.; Bian, X.; Yang, L.; Jing, X.J. Biodegradable electrospun fibers for drug delivery. J. Control Release 2003, 92, 227–231. [Google Scholar] [CrossRef]
- Kim, B.-Y.; Doh, H.-J.; Le, T.N.; Cho, W.-J.; Yong, C.-S.; Choi, H.-G.; Kim, J.S.; Lee, C.-H.; Kim, D.-D. Ketorolac amide prodrugs for transdermal delivery: Stability and in vitro rat skin permeation studies. Int. J. Pharm. 2005, 293, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Sill, T.; Von Recum, H.J.B. Electrospinning for tissue engineering and drug delivery. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Ma, G.; Fang, D.; Liu, Y.; Zhu, X.; Nie, J. Electrospun sodium alginate/poly(ethylene oxide) core–shell nanofibers scaffolds potential for tissue engineering applications. Carbohydr. Polym. 2012, 87, 737–743. [Google Scholar] [CrossRef]
- Seulmini Goh, D.K.; Choi, M.-H.; Shin, H.-J.; Kwon, S. Effects of Bamboo Stem Extracts on Adipogenic Differentiation and Lipid Metabolism Regulating Genes. Biotechnol. Bioprocess Eng. 2019, 24, 454–463. [Google Scholar] [CrossRef]
- Jayachandran, V.; Rekha, P.D.; Sukumaran, A.; Ira, B.; Sudha, P.N.; Chutiwan, D.; Se-Kwon, K.; Min Suk, S. Hydroxyapatite from Cuttlefish Bone: Isolation, Characterizations, and Applications. Biotechnol. Bioprocess Eng. 2018, 23, 383–393. [Google Scholar]
- Min, S.K.; Shim, H.J.; Shin, H.S. 3D Astrogliosis Model with bFGF and GFAP Expression Profiles Corresponding to an MCAO-injured Brain. Biotechnol. Bioprocess Eng. 2018, 23, 588–597. [Google Scholar] [CrossRef]
- Ashjari, H.R.; Ahmadi, A.; Dorraji, M.S.S. Synthesis and employment of PEGDA for fabrication of superhydrophilic PVDF/PEGDA electrospun nanofibrous membranes by in-situ visible photopolymerization. Korean J. Chem. Eng. 2018, 35, 289–297. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, M.; Mamman, K.; Saraf, S. Synthesis of fast swelling superporous hydrogel: Effect of concentration of crosslinker and acdisol on swelling ratio and mechanical strength. Int. J. Drug Deliv. 2011, 2. [Google Scholar] [CrossRef]
- Zhang, R.; Lei, L.; Song, Q.; Li, X. Calcium ion cross-linking alginate/dexamethasone sodium phosphate hybrid hydrogel for extended drug release. Colloids Surf. B Biointerfaces 2019, 175, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, N.; Galbis, E.; Valencia, C.; De-Paz, M.V.; Galbis, J.A. Reversible pH-Sensitive Chitosan-Based Hydrogels. Influence of Dispersion Composition on Rheological Properties and Sustained Drug Delivery. Polymers 2018, 10, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huebsch, N.; Mooney, D.J. Inspiration and application in the evolution of biomaterials. Nature 2009, 462, 426–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where We Have Been and Where We Are Going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef] [PubMed]
- Technical Evaluation Report: Alginate Handling/Processing; US Department of Agriculture: Washington, DC, USA, 2015; pp. 1–23.
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Gombotz, W.R.; Wee, S. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [CrossRef]
- Kim, B.; Choi, Y.; Choi, J.; Shin, Y.; Lee, S. Effect of surfactant on wetting due to fouling in membrane distillation membrane: Application of response surface methodology (RSM) and artificial neural networks (ANN). Korean J. Chem. Eng. 2020, 37, 1–10. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.-C. Monoolein cubosomes for enhancement of in vitro anti-oxidative efficacy of Bambusae Caulis in Taeniam extract toward carcinogenic fine dust-stimulated RAW 264.7 cells. Korean J. Chem. Eng. 2019, 36, 1466–1473. [Google Scholar] [CrossRef]
- Becker, T.A.; Kipke, D.R.; Brandon, T. Calcium alginate gel: A biocompatible and mechanically stable polymer for endovascular embolization. J. Biomed. Mater. Res. 2001, 54, 76–86. [Google Scholar] [CrossRef]
- Bondar, O.V.; Saifullina, D.; Shakhmaeva, I.; Mavlyutova, I.; Abdullin, T.J. Monitoring of the zeta potential of human cells upon reduction in their viability and interaction with polymers. Acta Naturae 2012, 4, 78–81. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef]
- Bonino, C.A.; Krebs, M.D.; Saquing, C.D.; Jeong, S.I.; Shearer, K.L.; Alsberg, E.; Khan, S.A. Electrospinning alginate-based nanofibers: From blends to crosslinked low molecular weight alginate-only systems. Carbohydr. Polym. 2011, 85, 111–119. [Google Scholar] [CrossRef]
- Bonino, C.A.; Efimenko, K.; Jeong, S.I.; Krebs, M.D.; Alsberg, E.; Khan, S.A. Three-Dimensional Electrospun Alginate Nanofiber Mats via Tailored Charge Repulsions. Small 2012, 8, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Li, Z.; Edmondson, D.; Zhang, M. Alginate-Based Nanofibrous Scaffolds: Structural, Mechanical, and Biological Properties. Adv. Mater. 2006, 18, 1463–1467. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Chi, H.; Wang, S. An alternative choice of lidocaine-loaded liposomes: Lidocaine-loaded lipid–polymer hybrid nanoparticles for local anesthetic therapy. Drug Deliv. 2016, 23, 1254–1260. [Google Scholar] [CrossRef] [Green Version]
- Choi, G.J.; Kang, H.; Hong, M.E.; Shin, H.Y.; Baek, C.W.; Jung, Y.H.; Lee, Y.; Kim, J.W.; Park, I.L.K.; Cho, W.J. Effects of a Lidocaine-Loaded Poloxamer/Alginate/CaCl2 Mixture on Postoperative Pain and Adhesion in a Rat Model of Incisional Pain. Anesth. Analg. 2017, 125, 320–327. [Google Scholar] [CrossRef]









| Final Concentration (in 10 mL of 2% Lidocaine Solution) | Formulation | ||
|---|---|---|---|
| Sodium Alginate | CMC 1 | PEO 2 | |
| 5% (w/v) | 225 mg | 225 mg | 50 mg |
| 7% (w/v) | 315 mg | 315 mg | 70 mg |
| 9% (w/v) | 405 mg | 405 mg | 90 mg |
| Concentration of CaCl2 (in 10 mL of 95% Ethanol) | ||
|---|---|---|
| 1% (w/v) | 3% (w/v) | 5% (w/v) |
| Electrospun Film Condition | Crosslinked with CaCl2 Concentration |
|---|---|
| 9% (w/v) alginate/CMC/PEO nanofiber film | 1% (w/v) CaCl2 |
| 3% (w/v) CaCl2 | |
| 5% (w/v) CaCl2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.; Park, H.; Park, Y.; Kang, H.; Lee, D. Development of a Lidocaine-Loaded Alginate/CMC/PEO Electrospun Nanofiber Film and Application as an Anti-Adhesion Barrier. Polymers 2020, 12, 618. https://doi.org/10.3390/polym12030618
Baek S, Park H, Park Y, Kang H, Lee D. Development of a Lidocaine-Loaded Alginate/CMC/PEO Electrospun Nanofiber Film and Application as an Anti-Adhesion Barrier. Polymers. 2020; 12(3):618. https://doi.org/10.3390/polym12030618
Chicago/Turabian StyleBaek, Seungho, Heekyung Park, Youngah Park, Hyun Kang, and Donghyun Lee. 2020. "Development of a Lidocaine-Loaded Alginate/CMC/PEO Electrospun Nanofiber Film and Application as an Anti-Adhesion Barrier" Polymers 12, no. 3: 618. https://doi.org/10.3390/polym12030618
APA StyleBaek, S., Park, H., Park, Y., Kang, H., & Lee, D. (2020). Development of a Lidocaine-Loaded Alginate/CMC/PEO Electrospun Nanofiber Film and Application as an Anti-Adhesion Barrier. Polymers, 12(3), 618. https://doi.org/10.3390/polym12030618