In Situ Enhanced Raman and Photoluminescence of Bio-Hybrid Ag/Polymer Nanoparticles by Localized Surface Plasmon for Highly Sensitive DNA Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. DNA Hybridizations
2.3. Measurement
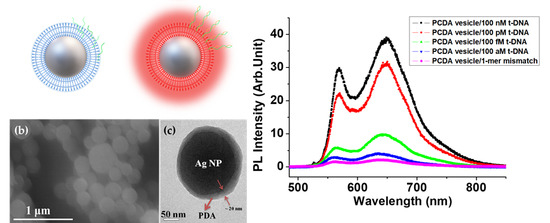
3. Results and Discussion
3.1. Ag/PDA Hybrid NPs and Probe DNA
3.2. Polymerization of PCDA into PDA
3.3. LSP Induced PL Enhancement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Novotny, L.; Stranick, S.J. Near-Field Optical Microscopy and Spectroscopy with Pointed Probes. Annu. Rev. Phys. Chem. 2006, 57, 303–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.H.; Kim, M.S.; Joo, J. Hybrid Nanostructures using Π-Conjugated Polymers and Nanoscale Metals: Synthesis, Characteristics, and Optoelectronic Applications. Chem. Soc. Rev. 2010, 39, 2439–2452. [Google Scholar] [CrossRef] [PubMed]
- Haran, G. Single-Molecule Raman Spectroscopy: A Probe of Surface Dynamics and Plasmonic Fields. Acc. Chem. Res. 2010, 43, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Watts, B.; Schuettfort, T.; McNeill, C.R. Mapping of Domain Orientation and Molecular Order in Polycrystalline Semiconducting Polymer Films with Soft X-Ray Microscopy. Adv. Funct. Mater. 2011, 21, 1122–1131. [Google Scholar] [CrossRef]
- McNeill, C.R.; Frohne, H.; Holdsworth, J.L.; Furst, J.E.; King, B.V.; Dastoor, P.C. Direct Photocurrent Mapping of Organic Solar Cells using a Near-Field Scanning Optical Microscope. Nano Lett. 2004, 4, 219–223. [Google Scholar] [CrossRef]
- Lim, D.; Jeon, K.; Kim, H.M.; Nam, J.; Suh, Y.D. Nanogap-Engineerable Raman-Active Nanodumbbells for Single-Molecule Detection. Nat. Mater. 2010, 9, 60–67. [Google Scholar] [CrossRef]
- Park, D.H.; Hong, Y.K.; Cho, E.H.; Kim, M.S.; Kim, D.; Bang, J.; Kim, J.; Joo, J. Light-Emitting Color Barcode Nanowires using Polymers: Nanoscale Optical Characteristics. ACS Nano 2010, 4, 5155–5162. [Google Scholar] [CrossRef]
- Park, D.H.; Jo, S.G.; Hong, Y.K.; Cui, C.; Lee, H.; Ahn, D.J.; Kim, J.; Joo, J. Highly Bright and Sharp Light Emission of a Single Nanoparticle of Crystalline Rubrene. J. Mater. Chem. 2011, 21, 8002–8007. [Google Scholar] [CrossRef]
- Hong, Y.K.; Park, D.H.; Jo, S.G.; Koo, M.H.; Kim, D.; Kim, J.; Kim, J.; Jang, S.; Joo, J. Fine Characteristics Tailoring of Organic and Inorganic Nanowires using Focused Electron-Beam Irradiation. Angew. Chem. Int. Ed. 2011, 50, 3734–3738. [Google Scholar] [CrossRef]
- Ozbay, E. Plasmonics: Merging Photonics and Electronics at Nanoscale Dimensions. Science 2006, 311, 189–193. [Google Scholar] [CrossRef]
- Barnes, W.L.; Dereux, A.; Ebbesen, T.W. Surface Plasmon Subwavelength Optics. Nature 2003, 424, 824–830. [Google Scholar] [CrossRef]
- Brillante, A.; Bilotti, I.; Della Valle, R.G.; Venuti, E.; Girlando, A. Probing Polymorphs of Organic Semiconductors by Lattice Phonon Raman Microscopy. CrystEngComm 2008, 10, 937–946. [Google Scholar] [CrossRef]
- Dellepiane, G.; Cuniberti, C.; Alloisio, M.; Demartini, A. Spectroscopical Properties of Organic/Metal Nanohybrids. Phys. Chem. Chem. Phys. 2010, 12, 2968–2974. [Google Scholar] [CrossRef] [PubMed]
- Atwater, H.A.; Polman, A. Plasmonics for Improved Photovoltaic Devices. Nat. Mater. 2010, 9, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Nie, S.M. Single-Molecule and Single-Nanoparticle SERS: From Fundamental Mechanisms to Biomedical Applications. Chem. Soc. Rev. 2008, 37, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Park, D.H.; Jeong, M.; Lee, Y.B.; Kim, H.S.; Choi, W.J.; Park, Q.; Kim, H.; Kim, D.; Kim, J. Bright Light Emission of a Single Polythiophene Nanotube Strand with a Nanometer-scale Metal Coating. Adv. Mater. 2007, 19, 2824–2829. [Google Scholar] [CrossRef]
- Liu, G.L.; Yin, Y.; Kunchakarra, S.; Mukherjee, B.; Gerion, D.; Jett, S.D.; Bear, D.G.; Gray, J.W.; Alivisatos, A.P.; Lee, L.P.; et al. A Nanoplasmonic Molecular Ruler for Measuring Nuclease Activity and DNA Footprinting. Nat. Nanotechnol. 2006, 1, 47–52. [Google Scholar] [CrossRef]
- Hu, M.; Chen, J.; Li, Z.; Au, L.; Hartland, G.V.; Li, X.; Marquez, M.; Xia, Y. Gold Nanostructures: Engineering their Plasmonic Properties for Biomedical Applications. Chem. Soc. Rev. 2006, 35, 1084–1094. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [Green Version]
- Gramotnev, D.K.; Bozhevolnyi, S.I. Plasmonics Beyond the Diffraction Limit. Nat. Photonics 2010, 4, 83–91. [Google Scholar] [CrossRef]
- Eustis, S.; El-Sayed, M.A. Why Gold Nanoparticles are More Precious than Pretty Gold: Noble Metal Surface Plasmon Resonance and its Enhancement of the Radiative and Nonradiative Properties of Nanocrystals of Different Shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Pelton, M.; Aizpurua, J.; Bryant, G. Metal-nanoparticle Plasmonics. Laser Photonics Rev. 2008, 2, 136–159. [Google Scholar] [CrossRef] [Green Version]
- Kinkhabwala, A.; Yu, Z.; Fan, S.; Avlasevich, Y.; Müllen, K.; Moerner, W.E. Large Single-Molecule Fluorescence Enhancements Produced by a Bowtie Nanoantenna. Nat. Photonics 2009, 3, 654–657. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Dadosh, T.; Sperling, J.; Bryant, G.W.; Breslow, R.; Shegai, T.; Dyshel, M.; Haran, G.; Bar-Joseph, I. Plasmonic Control of the Shape of the Raman Spectrum of a Single Molecule in a Silver Nanoparticle Dimer. ACS Nano 2009, 3, 1988–1994. [Google Scholar] [CrossRef]
- Lajos, G.; Jancura, D.; Miskovsky, P.; García-Ramos, J.V.; Sanchez-Cortes, S. Surface-Enhanced Fluorescence and Raman Scattering Study of Antitumoral Drug Hypericin: An Effect of Aggregation and Self-Spacing Depending on pH. J. Phys. Chem. C 2008, 112, 12974–12980. [Google Scholar] [CrossRef] [Green Version]
- Lordan, F.; Rice, J.H.; Jose, B.; Forster, R.J.; Keyes, T.E. Surface Enhanced Resonance Raman and Luminescence on Plasmon Active Nanostructured Cavities. Appl. Phys. Lett. 2010, 97, 153110. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, D.; Back, S. Demonstration of High Lateral Resolution in Laser Confocal Microscopy using Annular and Radially Polarized Light. Microsc. Res. Tech. 2009, 72, 441–446. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.; Kim, R.; Kim, J.; Park, D.; Kim, H.; Joo, J.; Suh, Y.D. Confocal Raman Spectroscopy of Single Poly (3-Methylthiophene) Nanotubes. J. Appl. Phys. 2007, 101, 053514. [Google Scholar] [CrossRef]
- Park, H.; Lee, J.; Choi, H.; Ahn, D.J.; Kim, J. Rational Design of Supramolecular Conjugated Polymers Displaying Unusual Colorimetric Stability upon Thermal Stress. Adv. Funct. Mater. 2007, 17, 3447–3455. [Google Scholar] [CrossRef]
- Ahn, D.J.; Kim, J. Fluorogenic Polydiacetylene Supramolecules: Immobilization, Micropatterning, and Application to Label-Free Chemosensors. Acc. Chem. Res. 2008, 41, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.; Kim, J. Polydiacetylene Liposome Arrays for Selective Potassium Detection. J. Am. Chem. Soc. 2008, 130, 5010–5011. [Google Scholar] [CrossRef] [PubMed]
- Reppy, M.A.; Pindzola, B.A. Biosensing with Polydiacetylene Materials: Structures, Optical Properties and Applications. Chem. Commun. 2007, 42, 4317–4338. [Google Scholar] [CrossRef] [PubMed]
- Nishide, J.; Oyamada, T.; Akiyama, S.; Sasabe, H.; Adachi, C. High Field-Effect Mobility in an Organic Thin-Film Transistor with a Solid-State Polymerized Polydiacetylene Film as an Active Layer. Adv. Mater. 2006, 18, 3120–3124. [Google Scholar] [CrossRef]
- Giorgetti, E.; Muniz-Miranda, M.; Margheri, G.; Giusti, A.; Sottini, S.; Alloisio, M.; Cuniberti, C.; Dellepiane, G. UV Polymerization of Self-Assembled Monolayers of a Novel Diacetylene on Silver: A Spectroscopic Analysis by Surface Plasmon Resonance and Surface Enhanced Raman Scattering. Langmuir 2006, 22, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Exarhos, G.J.; Risen, W.M., Jr.; Baughman, R.H. Resonance Raman Study of the Thermochromic Phase Transition of a Polydiacetylene. J. Am. Chem. Soc. 1976, 98, 481–487. [Google Scholar] [CrossRef]
- Back, S.H.; Park, J.H.; Cui, C.; Ahn, D.J. Bio-Recognitive Photonics of a DNA-Guided Organic Semiconductor. Nat. Commun. 2016, 7, 10234. [Google Scholar] [CrossRef]
- Cui, C.; Park, D.H.; Choi, H.; Joo, J.; Ahn, D.J. Protein Recognition by Phase Transition of Aptamer-Linked Polythiophene Single Nanowire. Small 2016, 12, 1154–1158. [Google Scholar] [CrossRef]
- Cui, C.; Kim, S.; Ahn, D.J.; Joo, J.; Lee, G.S.; Park, D.H.; Kim, B.-H. Unusual enhancement of fluorescence and Raman scattering of core-shell nanostructure of polydiacetylene and Ag nanoparticle. Synth. Met. 2018, 236, 19–23. [Google Scholar] [CrossRef]
- Guo, X. Surface Plasmon Resonance Based Biosensor Technique: A Review. J. Biophotonics 2012, 5, 483–501. [Google Scholar] [CrossRef]
- Yeom, S.; Kang, B.; Kim, K.; Kang, S. Nanostructures in Biosensor—A Review. Front. Biosci. 2011, 16, 997–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Park, D.H.; Cho, E.H.; Kim, K.H.; Park, Q.; Song, H.; Kim, D.; Kim, J.; Joo, J. Complex Nanoparticle of Light-Emitting MEH-PPV with Au: Enhanced Luminescence. ACS Nano 2009, 3, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Demartini, A.; Alloisio, M.; Cuniberti, C.; Dellepiane, G.; Jadhav, S.A.; Thea, S.; Giorgetti, E.; Gellini, C.; Muniz-Miranda, M. Polydiacetylene-Functionalized Noble Metal Nanocages. J. Phys. Chem. C 2009, 113, 19475–19481. [Google Scholar] [CrossRef]
- Gu, Z.; Zambrano, R.; McDermott, A. Hydrogen Bonding of Carboxyl Groups in Solid-State Amino Acids and Peptides Comparison of Carbon Chemical Shielding, Infrared Frequencies, and Structures. J. Am. Chem. Soc. 1994, 116, 6368–6372. [Google Scholar] [CrossRef]




| C–C Stretching Mode | C=C Stretching Mode | C≡C Stretching Mode | |
|---|---|---|---|
| PDA NP (blue phase) (a) | 695 cm−1 | 1451 cm−1 | 2072 cm−1 |
| Core-shell Ag/PDA NP (b) | 695 cm−1 | 1451 cm−1 (blue phase), 1477 cm−1 (red phase) | 2076 cm−1 |
| Enhancement Ratio of Intensities (case b/case a) | 83 | 92 | 78 |
| Normalized LCM Raman Intensity wrt C=C | 0.51 | 1.0 | 0.35 |
| 100 nM | 100 pM | 100 fM | 100 aM | |
|---|---|---|---|---|
| Intensity at 570 nm (a. u.) | 29.5 | 22.1 | 5.9 | 2.9 |
| Intensity at 648 nm (a. u.) | 38.5 | 30.8 | 9.3 | 4.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, B.-H.; Hong, Y.K.; Cui, C.; Choi, J.; Park, D.H.; Song, S.H. In Situ Enhanced Raman and Photoluminescence of Bio-Hybrid Ag/Polymer Nanoparticles by Localized Surface Plasmon for Highly Sensitive DNA Sensors. Polymers 2020, 12, 631. https://doi.org/10.3390/polym12030631
Kim S, Kim B-H, Hong YK, Cui C, Choi J, Park DH, Song SH. In Situ Enhanced Raman and Photoluminescence of Bio-Hybrid Ag/Polymer Nanoparticles by Localized Surface Plasmon for Highly Sensitive DNA Sensors. Polymers. 2020; 12(3):631. https://doi.org/10.3390/polym12030631
Chicago/Turabian StyleKim, Seokho, Bo-Hyun Kim, Young Ki Hong, Chunzhi Cui, Jinho Choi, Dong Hyuk Park, and Sung Ho Song. 2020. "In Situ Enhanced Raman and Photoluminescence of Bio-Hybrid Ag/Polymer Nanoparticles by Localized Surface Plasmon for Highly Sensitive DNA Sensors" Polymers 12, no. 3: 631. https://doi.org/10.3390/polym12030631
APA StyleKim, S., Kim, B. -H., Hong, Y. K., Cui, C., Choi, J., Park, D. H., & Song, S. H. (2020). In Situ Enhanced Raman and Photoluminescence of Bio-Hybrid Ag/Polymer Nanoparticles by Localized Surface Plasmon for Highly Sensitive DNA Sensors. Polymers, 12(3), 631. https://doi.org/10.3390/polym12030631