Robust Silica-Cellulose Composite Aerogels with a Nanoscale Interpenetrating Network Structure Prepared Using a Streamlined Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
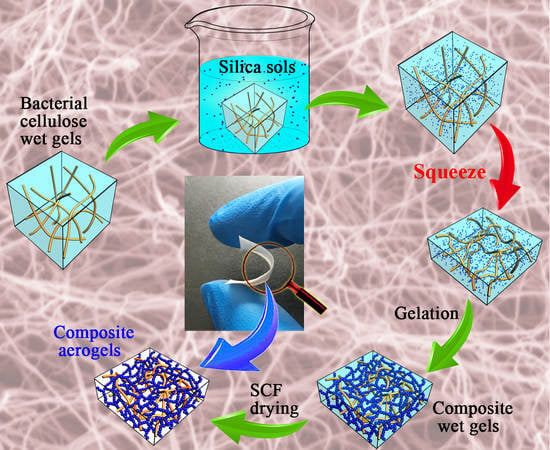
2.2. Preparation of the Bacterial Cellulose (BC) Wet Gel (i.e., Matrix)
2.3. Diffusion of Silica Alcosols in BC Wet Gel
2.4. Preparation of the Silica-Bacterial Cellulose Composite Aerogels
2.5. Characterizations
3. Results and Discussion
3.1. Diffusion of Silica Alcosols in BC Wet Gel
3.2. Microstructure of the Aerogels
3.3. Thermal Conductivity
3.4. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ziegler, C.; Wolf, A.; Liu, W.; Herrmann, A.-K.; Gaponik, N.; Eychmüller, A. Modern inorganic aerogels. Angew. Chem. Int. Ed. 2017, 56, 13200–13221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amonette, J.E.; Matyáš, J. Functionalized silica aerogels for gas-phase purification, sensing, and catalysis: A review. Microporous Mesoporous Mat. 2017, 250, 100–119. [Google Scholar] [CrossRef]
- Barrios, E.; Fox, D.; Li Sip, Y.Y.; Catarata, R.; Calderon, J.E.; Azim, N.; Afrin, S.; Zhang, Z.; Zhai, L. Nanomaterials in advanced, high-performance aerogel composites: A review. Polymers 2019, 11, 726. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wang, Z.; Liu, Z.; Cheng, H.; Li, C. Continuous, strong, porous silk firoin-based aerogel fibers toward textile thermal insulation. Polymers 2019, 11, 1899. [Google Scholar] [CrossRef] [Green Version]
- Hostler, S.R.; Abramson, A.R.; Gawryla, M.D.; Bandi, S.A.; Schiraldi, D.A. Thermal conductivity of a clay-based aerogel. Int. J. Heat Mass Transf. 2009, 52, 665–669. [Google Scholar] [CrossRef]
- Yagoub, H.; Zhu, L.; Shibraen, M.H.M.A.; Altam, A.A.; Babiker, D.M.D.; Liang, S.; Jin, Y.; Yang, S. Complex aerogels generated from nano-polysaccharides and its derivatives for oil–water separation. Polymers 2019, 11, 1593. [Google Scholar] [CrossRef] [Green Version]
- Bereczki, H.F.; Daróczi, L.; Fábián, I.; Lázár, I. Sol-gel synthesis, characterization and catalytic activity of silica aerogels functionalized with copper(II) complexes of cyclen and cyclam. Microporous Mesoporous Mat. 2016, 234, 392–400. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Q.; Li, Q.; Yu, H.; Liu, Y. Composite aerogels of carbon nanocellulose fibers and mixed-valent manganese oxides as renewable supercapacitor electrodes. Polymers 2019, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Alhwaige, A.A.; Ishida, H.; Qutubuddin, S. Carbon aerogels with excellent CO2 adsorption capacity synthesized from clay-reinforced biobased chitosan-polybenzoxazine nanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 1286–1295. [Google Scholar] [CrossRef]
- Quraishi, S.; Martins, M.; Barros, A.A.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Novel non-cytotoxic alginate–lignin hybrid aerogels as scaffolds for tissue engineering. J. Supercrit. Fluids 2015, 105, 1–8. [Google Scholar] [CrossRef]
- Hrubesh, L.W. Aerogel applications. J. Non-Cryst. Solids 1998, 225, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, Q.; Li, Y.; Zhang, Q.; Huang, J.; Wu, Q.; Wang, S. Fabrication of cellulose Nanocrystal-g-Poly(Acrylic Acid-Co-Acrylamide) aerogels for efficient Pb(II) removal. Polymers 2020, 12, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afrashi, M.; Semnani, D.; Talebi, Z.; Dehghan, P.; Maherolnaghsh, M. Comparing the drug loading and release of silica aerogel and PVA nano fibers. J. Non-Cryst. Solids 2019, 503–504, 186–193. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Zhang, G.; Rawashdeh, A.-M.M. Nanoengineering strong silica aerogels. Nano Letters 2002, 2, 957–960. [Google Scholar] [CrossRef]
- Patil, S.P.; Rege, A.; Itskov, M.; Markert, B. Fracture of silica aerogels: An all-atom simulation study. J. Non-Cryst. Solids 2018, 498, 125–129. [Google Scholar] [CrossRef]
- Garrido, R.; Silvestre, J.D.; Flores-Colen, I.; de Fátima Júlio, M.; Pedroso, M. Economic assessment of the production of subcritically dried silica-based aerogels. J. Non-Cryst. Solids 2019, 516, 26–34. [Google Scholar] [CrossRef]
- Ślosarczyk, A. Recent advances in research on the synthetic fiber based silica aerogel nanocomposites. Nanomaterials 2017, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Wu, X.; Guo, D.; Fang, J. Preparation of flexible, hydrophobic, and oleophilic silica aerogels based on a methyltriethoxysilane precursor. J. Mater. Sci. 2014, 49, 7715–7722. [Google Scholar] [CrossRef]
- Zu, G.; Kanamori, K.; Maeno, A.; Kaji, H.; Nakanishi, K. Superflexible multifunctional polyvinylpolydimethylsiloxane-based aerogels as efficient absorbents, thermal superinsulators, and strain sensors. Angew. Chem. Int. Ed. 2018, 57, 9722–9727. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Dai, Z.; Wu, J.; Zhao, N.; Xu, J. Vacuum-dried robust bridged silsesquioxane aerogels. Adv. Mater. 2013, 25, 4494–4497. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Girão, A.V.; Silva, R.F.; Durães, L. Polysilsesquioxane-based silica aerogel monoliths with embedded CNTs. Microporous Mesoporous Mat. 2019, 288, 109575. [Google Scholar] [CrossRef]
- Dervin, S.; Lang, Y.; Perova, T.; Hinder, S.H.; Pillai, S.C. Graphene oxide reinforced high surface area silica aerogels. J. Non-Cryst. Solids 2017, 465, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Feng, J.; Wang, X.; Luo, C.; Maloko Loussala, H.; Sun, M. An organic-inorganic hybrid silica aerogel prepared by co-precursor method for solid-phase microextraction coating. Talanta 2019, 194, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Zu, G.; Shimizu, T.; Kanamori, K.; Zhu, Y.; Maeno, A.; Kaji, H.; Shen, J.; Nakanishi, K. Transparent, Superflexible Doubly Cross-Linked Polyvinylpolymethylsiloxane aerogel superinsulators via ambient pressure drying. ACS Nano 2018, 12, 521–532. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Su, D.; Ji, H.; Wang, X. Ultra-low thermal conductivity and high strength of aerogels/fibrous ceramic composites. J. Eur. Ceram. Soc. 2016, 36, 1487–1493. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, J.; Ni, X.; Wu, G.; Zhou, B.; Yang, M.; Gu, X.; Qian, M.; Wu, Y. Hydrophobic silica aerogels strengthened with nonwoven fibers. J. Macromol. Sci. Part A 2006, 43, 1663–1670. [Google Scholar] [CrossRef]
- Markevicius, G.; Ladj, R.; Niemeyer, P.; Budtova, T.; Rigacci, A. Ambient-dried thermal superinsulating monolithic silica-based aerogels with short cellulosic fibers. J. Mater. Sci. 2017, 52, 2210–2221. [Google Scholar] [CrossRef]
- Shafi, S.; Navik, R.; Ding, X.; Zhao, Y. Improved heat insulation and mechanical properties of silica aerogel/glass fiber composite by impregnating silica gel. J. Non-Cryst. Solids 2019, 503–504, 78–83. [Google Scholar] [CrossRef]
- Huang, Y.; He, S.; Chen, G.; Dai, H.; Yuan, B.; Chen, X.; Yang, X. Fast preparation of glass fiber/silica aerogel blanket in ethanol & water solvent system. J. Non-Cryst. Solids 2019, 505, 286–291. [Google Scholar]
- Fei, Z.; Yang, Z.; Chen, G.; Li, K.; Zhao, S.; Su, G. Preparation and characterization of glass fiber/polyimide/SiO2 composite aerogels with high specific surface area. J. Mater. Sci. 2018, 53, 12885–12893. [Google Scholar] [CrossRef]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring mechanical properties of aerogels for aerospace applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef]
- Zheng, H.; Shan, H.; Bai, Y.; Wang, X.; Liu, L.; Yu, J.; Ding, B. Assembly of silica aerogels within silica nanofibers: Towards a super-insulating flexible hybrid aerogel membrane. RSC Adv. 2015, 5, 91813–91820. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, Y.; Tebyetekerwa, M.; Meng, S.; Zhu, M.; Lu, Y. “Stiff–Soft” binary synergistic aerogels with superflexibility and high thermal insulation performance. Adv. Funct. Mater. 2019, 29, 1806407. [Google Scholar] [CrossRef]
- Wang, X.; Jana, S.C. Synergistic hybrid organic–inorganic aerogels. ACS Appl. Mater. Interfaces 2013, 5, 6423–6429. [Google Scholar] [CrossRef]
- Mohite, D.P.; Larimore, Z.J.; Lu, H.; Mang, J.T.; Sotiriou-Leventis, C.; Leventis, N. Monolithic hierarchical fractal assemblies of silica nanoparticles cross-linked with polynorbornene via ROMP: A structure–property correlation from molecular to bulk through nano. Chem. Mater. 2012, 24, 3434–3448. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Z.; Sèbe, G.; Wu, R.; Rivera Virtudazo, R.V.; Tingaut, P.; Koebel, M.M. Multiscale assembly of superinsulating silica aerogels within silylated nanocellulosic scaffolds: Improved mechanical properties promoted by nanoscale chemical compatibilization. Adv. Funct. Mater. 2015, 25, 2326–2334. [Google Scholar] [CrossRef]
- Sai, H.; Fu, R.; Xiang, J.; Guan, Y.; Zhang, F. Fabrication of elastic silica-bacterial cellulose composite aerogels with nanoscale interpenetrating network by ultrafast evaporative drying. Compos. Sci. Technol. 2018, 155, 72–80. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose aerogels: Synthesis, applications, and prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [Green Version]
- Sai, H.; Xing, L.; Xiang, J.; Cui, L.; Jiao, J.; Zhao, C.; Li, Z.; Li, F. Flexible aerogels based on an interpenetrating network of bacterial cellulose and silica by a non-supercritical drying process. J. Mater. Chem. A 2013, 1, 7963–7970. [Google Scholar] [CrossRef]
- Sai, H.; Xing, L.; Xiang, J.; Cui, L.; Jiao, J.; Zhao, C.; Li, Z.; Li, F.; Zhang, T. Flexible aerogels with interpenetrating network structure of bacterial cellulose-silica composite from sodium silicate precursor via freeze drying process. RSC Adv. 2014, 4, 30453–30461. [Google Scholar] [CrossRef]
- Fu, J.; Wang, S.; He, C.; Lu, Z.; Huang, J.; Chen, Z. Facilitated fabrication of high strength silica aerogels using cellulose nanofibrils as scaffold. Carbohydr. Polym. 2016, 147, 89–96. [Google Scholar] [CrossRef] [PubMed]
- He, F.; He, X.; Yang, W.; Zhang, X.; Zhou, L. In-situ synthesis and structural characterization of cellulose-silica aerogels by one-step impregnation. J. Non-Cryst. Solids 2018, 488, 36–43. [Google Scholar] [CrossRef]
- Cai, J.; Liu, S.; Feng, J.; Kimura, S.; Wada, M.; Kuga, S.; Zhang, L. Cellulose-silica nanocomposite aerogels by in situ formation of silica in cellulose gel. Angew. Chem. Int. Ed. 2012, 51, 2076–2079. [Google Scholar] [CrossRef] [PubMed]
- Demilecamps, A.; Beauger, C.; Hildenbrand, C.; Rigacci, A.; Budtova, T. Cellulose–silica aerogels. Carbohydr. Polym. 2015, 122, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Shafi, S.; Zhao, Y. Preparation of compressible silica aerogel reinforced by bacterial cellulose using tetraethylorthosilicate and methyltrimethoxylsilane co-precursor. J. Non-Cryst. Solids 2018, 481, 622–626. [Google Scholar] [CrossRef]
- Sedighi Gilani, M.; Boone, M.N.; Fife, J.L.; Zhao, S.; Koebel, M.M.; Zimmermann, T.; Tingaut, P. Structure of cellulose -silica hybrid aerogel at sub-micron scale, studied by synchrotron X-ray tomographic microscopy. Compos. Sci. Technol. 2016, 124, 71–80. [Google Scholar] [CrossRef]
- Sarawade, P.B.; Kim, J.K.; Kim, H.K.; Kim, H.T. High specific surface area TEOS-based aerogels with large pore volume prepared at an ambient pressure. Appl. Surf. Sci. 2007, 254, 574–579. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Weber, A.S.; Hindi, A.; Naumenko, M.; McCorkle, L.; Quade, D.; Vivod, S.L.; Gould, G.L.; White, S.; Deshpande, K. Structure−property relationships in porous 3D nanostructures: Epoxy-cross-linked silica aerogels produced using ethanol as the solvent. ACS Appl. Mater. Interfaces 2009, 1, 894–906. [Google Scholar] [CrossRef]
- Zhang, G.H.; Dass, A.; Rawashdeh, A.M.M.; Thomas, J.; Counsil, J.A.; Sotiriou-Leventis, C.; Fabrizio, E.F.; Ilhan, F.; Vassilaras, P.; Scheiman, D.A.; et al. Isocyanate-crosslinked silica aerogel monoliths: Preparation and characterization. J. Non-Cryst. Solids 2004, 350, 152–164. [Google Scholar] [CrossRef]
- Zu, G.; Shen, J.; Wang, W.; Zou, L.; Lian, Y.; Zhang, Z. Silica–titania composite aerogel photocatalysts by chemical liquid deposition of titania onto nanoporous silica scaffolds. ACS Appl. Mater. Interfaces 2015, 7, 5400–5409. [Google Scholar] [CrossRef] [PubMed]
- Zu, G.; Shen, J.; Wang, W.; Zou, L.; Lian, Y.; Zhang, Z.; Liu, B.; Zhang, F. Robust, highly thermally stable, core–shell nanostructured metal oxide aerogels as high-temperature thermal superinsulators, adsorbents, and catalysts. Chem. Mater. 2014, 26, 5761–5772. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Aerogel insulation for building applications: A state-of-the-art review. Energy Build. 2011, 43, 761–769. [Google Scholar] [CrossRef] [Green Version]






| Materials | SiO2 in Aerogels [% w/w] | Bulk Density [g cm−3] | SBETa [m2 g−1] | Pore Volume (cm3 g−1) | D b [nm] | P c [%] | Thermal Conductivity [W m−1 K−1] |
|---|---|---|---|---|---|---|---|
| BM | 0 | 0.007 | 113 | 0.35 | 11.4 | 99.5 | 0.029 |
| CA-1 | 78 | 0.032 | 440 | 1.59 | 14.7 | 98.4 | 0.031 |
| CA-2 | 88 | 0.060 | 641 | 1.50 | 9.2 | 97.1 | 0.034 |
| CA-3 | 91 | 0.082 | 648 | 1.56 | 9.5 | 96.0 | 0.028 |
| CA-4 | 93 | 0.104 | 667 | 2.16 | 13.7 | 94.9 | 0.030 |
| CA-4/6 | 89 | 0.108 | 661 | 2.08 | 13.2 | 94.7 | 0.031 |
| CA-4/3 | 81 | 0.119 | 647 | 1.96 | 13.3 | 94.1 | 0.033 |
| Materials | Flexural Properties | Tensile Properties | |||
|---|---|---|---|---|---|
| Max Flex Stress (MPa) | Flexible Modulus (MPa) | Breaking Stress (MPa) | Elongation at Break (%) | Tensile Modulus (MPa) | |
| BM | 0.05 | 3.45 | 1.12 | 20.0 | 2.95 |
| CA-1 | 0.21 | 4.84 | 1.18 | 18.3 | 3.06 |
| CA-2 | 0.39 | 9.65 | 1.09 | 16.1 | 2.91 |
| CA-3 | 0.57 | 10.99 | 0.99 | 12.7 | 5.42 |
| CA-4 | 0.73 | 13.46 | 1.11 | 11.5 | 9.13 |
| CA-4/6 | 1.47 | 48.76 | 1.70 | 9.6 | 12.92 |
| CA-4/3 | 2.88 | 274.49 | 3.06 | 5.4 | 46.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sai, H.; Zhang, J.; Jin, Z.; Fu, R.; Wang, M.; Wang, Y.; Wang, Y.; Ma, L. Robust Silica-Cellulose Composite Aerogels with a Nanoscale Interpenetrating Network Structure Prepared Using a Streamlined Process. Polymers 2020, 12, 807. https://doi.org/10.3390/polym12040807
Sai H, Zhang J, Jin Z, Fu R, Wang M, Wang Y, Wang Y, Ma L. Robust Silica-Cellulose Composite Aerogels with a Nanoscale Interpenetrating Network Structure Prepared Using a Streamlined Process. Polymers. 2020; 12(4):807. https://doi.org/10.3390/polym12040807
Chicago/Turabian StyleSai, Huazheng, Jing Zhang, Zhiqiang Jin, Rui Fu, Meijuan Wang, Yutong Wang, Yaxiong Wang, and Litong Ma. 2020. "Robust Silica-Cellulose Composite Aerogels with a Nanoscale Interpenetrating Network Structure Prepared Using a Streamlined Process" Polymers 12, no. 4: 807. https://doi.org/10.3390/polym12040807
APA StyleSai, H., Zhang, J., Jin, Z., Fu, R., Wang, M., Wang, Y., Wang, Y., & Ma, L. (2020). Robust Silica-Cellulose Composite Aerogels with a Nanoscale Interpenetrating Network Structure Prepared Using a Streamlined Process. Polymers, 12(4), 807. https://doi.org/10.3390/polym12040807