Bio-Based Aromatic Copolyesters: Influence of Chemical Microstructures on Thermal and Crystalline Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. General Procedure for the Synthesis of Polyesters
3. Results and Discussion
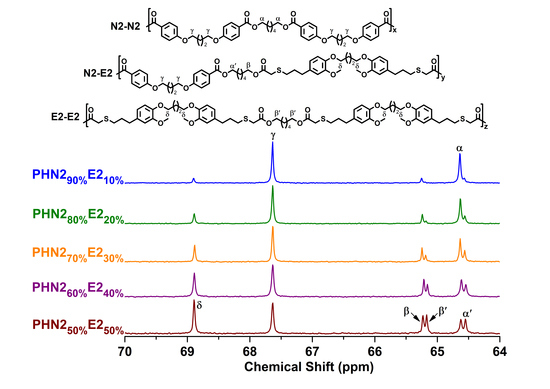
3.1. Synthesis and Structures of the Polyesters
3.2. Thermal Properties
3.3. Powder X-ray Diffraction Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, G.-Q.; Patel, M.K. Plastics derived from biological sources: Present and future: A technical and environmental review. Chem. Rev. 2012, 112, 2082–2099. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Artz, J.; Müller, T.E.; Thenert, K. Sustainable conversion of carbon dioxide: An integrated review of catalysis and life cycle assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef] [PubMed]
- Alexandratos, S.D.; Barak, N.; Bauer, D.; Davidson, F.T.; Gibney, B.R.; Hubbard, S.S.; Taft, H.L.; Westerhof, P. Sustaining water resources: Environmental and economic impact. ACS Sustainable Chem. Eng. 2019, 7, 2879–2888. [Google Scholar] [CrossRef] [Green Version]
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef] [PubMed]
- Laycock, B.; Nikolić, M.; Colwell, J.M.; Gauthier, E.; Halley, P.; Bottle, S.; George, G. Lifetime prediction of biodegradable polymers. Prog. Polym. Sci. 2017, 71, 144–189. [Google Scholar] [CrossRef] [Green Version]
- Tardy, A.; Nicolas, J.; Gigmes, D.; Lefay, C.; Guillaneuf, Y. Radical ring-opening polymerization: Scope, limitations, and application to (bio)degradable materials. Chem. Rev. 2017, 117, 1319–1406. [Google Scholar] [CrossRef]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.; Zhao, D.; Wu, G.; Ma, J. Synthesis and properties of polyesters derived from renewable eugenol and α,ω-diols via a continuous overheating method. Polym. Chem. 2015, 6, 7138–7148. [Google Scholar] [CrossRef]
- Hu, K.; Zhao, D.; Wu, G.; Ma, J. Toughened aromatic poly-(decylene terephthalate) copolyesters with two renewable eugenol-based components via a random copolymerization method. Polym. Chem. 2016, 7, 1096–1110. [Google Scholar] [CrossRef]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.-P. Biobased thermosetting epoxy: present and future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A.; Lacerda, T.M. From monomers to polymers from renewable resources: Recent advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, K.; Kai, D.; Li, Z.; Loh, X.J. Polyester elastomers for soft tissue engineering. Chem. Soc. Rev. 2018, 47, 4545–4580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ganewatta, M.S.; Tang, C. Sustainable polymers from biomass: Bridging chemistry with materials and processing. Prog. Polym. Sci. 2020, 101, 101197. [Google Scholar] [CrossRef]
- Li, Z.; Chueh, C.-C.; Jen, A.K.-Y. Recent advances in molecular design of functional conjugated polymers for high-performance polymer solar cells. Prog. Polym. Sci. 2019, 99, 101175. [Google Scholar] [CrossRef]
- Herzberger, J.; Sirrine, J.M.; Williams, C.B.; Long, T.E. Polymer design for 3D printing elastomers: Recent advances in structure, properties, and printing. Prog. Polym. Sci. 2019, 97, 101144. [Google Scholar] [CrossRef]
- Su, W.-F.A.; Chen, K.C.; Tseng, S.Y. Effects of chemical structure changes on thermal, mechanical, and crystalline properties of rigid rod epoxy resins. J. Appl. Polym. Sci. 2000, 78, 446–451. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Terzopoulou, Z.; Bikiaris, D.N. Production of bio-based 2,5-furan dicarboxylate polyesters: Recent progress and critical aspects in their synthesis and thermal properties. Eur. Polym. J. 2016, 83, 202–229. [Google Scholar] [CrossRef]
- McKiernan, R.L.; Cardoen, G.; Boutevin, B.; Améduri, B.; Gido, S.P.; Penelle, J. Macromolecular crystal engineering based on segmented polymers: Influence of heteroatoms on the thermal properties and crystallization of m,n-polyurethanes derived from long-chain, heteroatom-containing, monodisperse α,ω-diols. Macromol. Chem. Phys. 2003, 204, 961–969. [Google Scholar] [CrossRef]
- İçli-Özkut, M.; Mersini, J.; Önal, A.M.; Cihaner, A. Substituent and heteroatom effects on the electrochromic properties of similar systems. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 615–621. [Google Scholar] [CrossRef]
- Glassner, M.; Vergaelen, M.; Hoogenboom, R. Poly(2-oxazoline)s: A comprehensive overview of polymer structures and their physical properties. Polym. Int. 2018, 67, 32–45. [Google Scholar] [CrossRef]
- Mutlu, H.; Ceper, E.B.; Li, X.; Yang, J.; Dong, W.; Ozmen, M.M.; Theato, P. Sulfur chemistry in polymer and materials science. Macromol. Rapid Commun. 2019, 40, 1800650. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Polymer engineering based on reversible covalent chemistry: A promising innovative pathway towards new materials and new functionalities. Prog. Polym. Sci. 2018, 80, 39–93. [Google Scholar] [CrossRef]
- Fakirov, S. Condensation polymers: Their chemical peculiarities offer great opportunities. Prog. Polym. Sci. 2019, 89, 1–18. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Hillmyer, M.A.; Tolman, W.B. Aliphatic polyester block polymers: Renewable, degradable, and sustainable. Acc. Chem. Res. 2014, 47, 2390–2396. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- Miller, K.A.; Morado, E.G.; Samanta, S.R.; Walker, B.A.; Nelson, A.Z.; Sen, S.; Tran, D.T.; Whitaker, D.J.; Ewoldt, R.H.; Braun, P.V.; et al. Acid-triggered, acid-generating, and self-amplifying degradable polymers. J. Am. Chem. Soc. 2019, 141, 2838–2842. [Google Scholar] [CrossRef]
- Hsu, T.-G.; Zhou, J.; Su, H.-W.; Schrage, B.R.; Ziegler, C.J.; Wang, J. A polymer with “Locked” degradability: Superior backbone stability and accessible degradability enabled by mechanophore installation. J. Am. Chem. Soc. 2020, 142, 2100–2104. [Google Scholar] [CrossRef]
- Lavilla, C.; Gubbels, E.; Ilarduya, A.M.de.; Noordover, B.A.J.; Koning, C.E.; Muñoz-Guerra, S. Solid-state modification of PBT with cyclic acetalized galactitol and D-mannitol: Influence of composition and chemical microstructure on thermal properties. Macromolecules 2013, 46, 4335–4345. [Google Scholar] [CrossRef]
- Li, M.; Cui, F.; Li, Y.; Tao, Y.; Wang, X. Crystalline regio-/stereoregular glycine-bearing polymers from ROMP: Effect of microstructures on materials performances. Macromolecules 2016, 49, 9415–9424. [Google Scholar] [CrossRef]
- Tomizawa, S.; Chuah, J.-A.; Matsumoto, K.; Doi, Y.; Numata, K. Understanding the limitations in the biosynthesis of polyhydroxyalkanoate (PHA) from lignin derivatives. ACS Sustain. Chem. Eng. 2014, 2, 1106–1113. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jang, J. The effect of mesogenic length on the curing behavior and properties of liquid crystalline epoxy resins. Polymer 2006, 47, 3036–3042. [Google Scholar] [CrossRef]
- Taylor, J.E.; Romo-Uribe, A.; Libera, M.R. Bimodal orientation defects in main-chain thermotropic liquid crystalline polymer fibers. Macromolecules 2002, 35, 1751–1756. [Google Scholar] [CrossRef]
- Lummiss, J.A.M.; Oliveira, K.C.; Pranckevicius, A.M.T.; Santos, A.G.; dos Santos, E.N.; Fogg, D.E. Chemical plants: High-Value molecules from essential oils. J. Am. Chem. Soc. 2012, 134, 18889–18891. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, X.; Ji, Y.; Zhao, Z.; Li, W.; Jia, X. Sustainable Preparation of bio-based polybenzoxazine resins from amino acid and their application in CO2 adsorption. ACS Sustain. Chem. Eng. 2019, 7, 17313–17324. [Google Scholar] [CrossRef]
- Watanabe, H.; Takahashi, M.; Kihara, H.; Yoshida, M. Photocurable urushiol analogues bearing methacryloxy-containing side chains. Langmuir 2019, 35, 4534–4539. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol-ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Yearty, K.L.; Maynard, R.K.; Cortes, C.N.; Morrison, R.W. A multioutcome experiment for the Williamson ether synthesis. J. Chem. Educ. 2020, 97, 578–581. [Google Scholar] [CrossRef]
- Japu, C.; Alla, A.; Ilarduya, A.M.de.; García-Martín, M.G.; Benito, E.; Galbis, J.A.; Muñoz-Guerra, S. Bio-based aromatic copolyesters made from 1,6-hexanediol and bicyclic diacetalized D-glucitol. Polym. Chem. 2012, 3, 2092–2101. [Google Scholar] [CrossRef]
- Burt, S.P.; Barnett, K.J.; McClelland, D.J.; Wolf, P.; Dumesic, J.A.; Huber, G.W.; Hermans, I. Production of 1,6-hexanediol from tetrahydropyran-2-methanol by dehydration-hydration and hydrogenation. Green Chem. 2017, 19, 1390–1398. [Google Scholar] [CrossRef]
- Japu, C.; Ilarduya, A.M.de.; Alla, A.; García-Martín, M.G.; Galbis, J.A.; Muñoz-Guerra, S. D-Glucose-derived PET copolyesters with enhanced Tg. Polym. Chem. 2013, 4, 3524–3536. [Google Scholar] [CrossRef]
- Lavilla, C.; Gubbels, E.; Alla, A.; Ilarduya, A.M.de.; Noordover, B.A.J.; Koning, C.E.; Muñoz-Guerra, S. Carbohydrate-based PBT copolyesters from a cyclic diol derived from naturally occurring tartaric acid: A comparative study regarding melt polycondensation and solid-state modification. Green Chem. 2014, 16, 1789–1798. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.; Wu, G.; Ma, J. Aromatic copolyesters with enhanced crystallizability and mechanical properties byadding the renewable nipagin-based composition. RSC Adv. 2016, 6, 21555–21563. [Google Scholar] [CrossRef]
- Tanzer, J.D.; Bartels, C.R.; Crist, B. Dimensions of polymer chains in the semicrystalline solid state. Macromolecules 1984, 17, 2708–2714. [Google Scholar] [CrossRef]
- Tsao, H.-K.; Chen, J.Z.Y.; Sheng, Y.-J. Effect of intrachain mismatch on loop-to-coil transition of an associating chain. Macromolecules 2003, 36, 5863–5872. [Google Scholar] [CrossRef]
- Hisano, M.; Takeda, K.; Takashima, T.; Jin, Z.; Shiibashi, A.; Matsumoto, A. Sequence-controlled radical copolymerization of N-substituted maleimides with olefins and polyisobutene macromonomers to fabricate thermally stable and transparent maleimide copolymers with tunable glass transition temperatures and viscoelastic properties. Macromolecules 2013, 46, 7733–7744. [Google Scholar] [CrossRef]
- Mazidi, M.M.; Edalat, A.; Berahman, R.; Hosseini, F.S. Highly-toughened polylactide-(PLA-) based ternary blends with significantly enhanced glass transition and melt strength: Tailoring the interfacial interactions, phase morphology, and performance. Macromolecules 2018, 51, 4298–4314. [Google Scholar] [CrossRef]
- Shen, Z.; Luo, F.; Bai, H.; Si, P.; Lei, X.; Ding, S.; Ji, L. A study on mediating the crystallization behavior of PBT through intermolecular hydrogen-bonding. RSC Adv. 2016, 6, 17510–17518. [Google Scholar] [CrossRef]
- Japu, C.; de Ilarduya, A.M.; Alla, A.; García-Martín, M.G.; Galbis, J.A.; Muñoz-Guerra, S. Bio-based PBT copolyesters derived from D-glucose: Influence of composition on properties. Polym. Chem. 2014, 5, 3190–3202. [Google Scholar] [CrossRef]









| Copolyester | Yield (%) | Molar Composition | Molecular Weight | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Feed Ratio | Final Product 3 | ||||||||
| XN2 | XE | XN2 | XE | Mn | Mw | D | |||
| PHN2 1 | 86% | 100 | 0 | 100 | 0 | 14200 | 24300 | 1.7 | |
| PHN290%E110% 1 | 83% | 90 | 10 | 89.3 | 10.7 | 18600 | 35900 | 1.9 | |
| PHN280%E120% 1 | 85% | 80 | 20 | 80.6 | 19.4 | 19200 | 37800 | 2.0 | |
| PHN270%E130% 1 | 87% | 70 | 30 | 70.8 | 29.2 | 20900 | 42100 | 2.0 | |
| PHN260%E140% 2 | 81% | 60 | 40 | 62.5 | 37.5 | 15100 | 28300 | 1.9 | |
| PHN250%E150% 2 | 85% | 50 | 50 | 53.2 | 46.8 | 19900 | 35400 | 1.8 | |
| PHN290%E210% 1 | 82% | 90 | 10 | 89.3 | 10.7 | 19800 | 39300 | 2.0 | |
| PHN280%E220% 1 | 87% | 80 | 20 | 80.0 | 20.0 | 20400 | 40200 | 2.0 | |
| PHN270%E230% 2 | 86% | 70 | 30 | 69.9 | 30.1 | 20400 | 37500 | 1.8 | |
| PHN260%E240% 2 | 89% | 60 | 40 | 59.9 | 40.1 | 14300 | 25900 | 1.8 | |
| PHN250%E250% 2 | 88% | 50 | 50 | 49.8 | 50.2 | 21000 | 37400 | 1.8 | |
| Polyester | TGA | DSC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T5%1 (°C) | Td2 (°C) | W3 (%) | First Cooling 4 | Second Heating 5 | |||||||
| Tc (°C) | ΔHc (J g−1) | Tg (°C) | Tc (°C) | ΔHc (J g−1) | Tm (°C) | ΔHm (J g−1) | |||||
| PHN2 | 395 | 432/614 | 2.1 | 131.0 | 163.8 | 32.2 | - | - | Tm1 = 152.4 Tm2 = 160.3 | ΔHm1 = 16.5 ΔHm2 = 147.8 | |
| PHN290%E110% | 388 | 422 | 17.5 | 118.4 | 139.2 | 23.2 | - | - | Tm1 = 144.3 Tm2 = 154.3 | ΔHm1 = 8.3 ΔHm2 = 130.5 | |
| PHN280%E120% | 381 | 419 | 18.6 | 105.9 | 109.2 | 12.5 | - | - | 144.3 | 108.7 | |
| PHN270%E130% | 374 | 418 | 19.3 | 83.3 | 78.9 | 6.3 | - | - | 134.3 | 79.2 | |
| PHN260%E140% | 367 | 417 | 17.0 | 63.3 | 54.3 | 0.5 | - | - | 124.2 | 54.6 | |
| PHN250%E150% | 365 | 408 | 15.8 | 30.8 | 28.2 | -5.8 | - | - | 111.7 | 28.5 | |
| PHN290%E210% | 387 | 420 | 3.9 | 115.9 | 123.8 | 18.6 | - | - | 151.8 | 123.4 | |
| PHN280%E220% | 384 | 420 | 5.3 | 98.4 | 98.7 | 6.4 | - | - | 141.8 | 99.2 | |
| PHN270%E230% | 377 | 420 | 15.4 | 73.3 | 71.3 | 1.8 | - | - | 129.3 | 70.9 | |
| PHN260%E240% | 378 | 412 | 13.4 | 35.8 | 49.1 | -0.5 | - | - | 119.2 | 48.7 | |
| PHN250%E250% | 376 | 410 | 12.1 | - | - | -2.4 | 34.6 | 26.3 | 106.6 | 25.8 | |
| Polyester | X-ray Diffraction Data | ||||
|---|---|---|---|---|---|
| 2θ (°) 1 | Xc2 | ||||
| PHN2 | 16.00 m | 16.88 m | 21.02 w | 24.56 s | 0.22 |
| PHN290%E110% | 16.00 m | 16.88 m | 21.02 w | 24.56 s | 0.19 |
| PHN280%E120% | 16.00 m | 16.88 m | 21.02 w | 24.56 s | 0.18 |
| PHN270%E130% | 16.00 m | 16.88 m | 21.02 w | 24.56 s | 0.16 |
| PHN260%E140% | 16.00 w | 16.88 w | 21.02 m | 24.56 s | 0.15 |
| PHN250%E150% | 16.00 w | 16.88 w | 21.02 w | 24.56 m | 0.18 |
| PHN290%E210% | 16.00 m | 16.88 m | 21.02 w | 24.56 s | 0.16 |
| PHN280%E220% | 16.00 m | 16.88 m | 21.02 w | 24.56 s | 0.15 |
| PHN270%E230% | 16.00 w | 16.88 w | 21.02 m | 24.56 s | 0.13 |
| PHN260%E240% | 16.00 w | 16.88 w | 21.02 w | 24.56 m | 0.11 |
| PHN250%E250% | - | - | - | - | - |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, K. Bio-Based Aromatic Copolyesters: Influence of Chemical Microstructures on Thermal and Crystalline Properties. Polymers 2020, 12, 829. https://doi.org/10.3390/polym12040829
Hu K. Bio-Based Aromatic Copolyesters: Influence of Chemical Microstructures on Thermal and Crystalline Properties. Polymers. 2020; 12(4):829. https://doi.org/10.3390/polym12040829
Chicago/Turabian StyleHu, Keling. 2020. "Bio-Based Aromatic Copolyesters: Influence of Chemical Microstructures on Thermal and Crystalline Properties" Polymers 12, no. 4: 829. https://doi.org/10.3390/polym12040829
APA StyleHu, K. (2020). Bio-Based Aromatic Copolyesters: Influence of Chemical Microstructures on Thermal and Crystalline Properties. Polymers, 12(4), 829. https://doi.org/10.3390/polym12040829