Tuning Functional Behavior of Humic Acids through Interactions with Stöber Silica Nanoparticles
Abstract
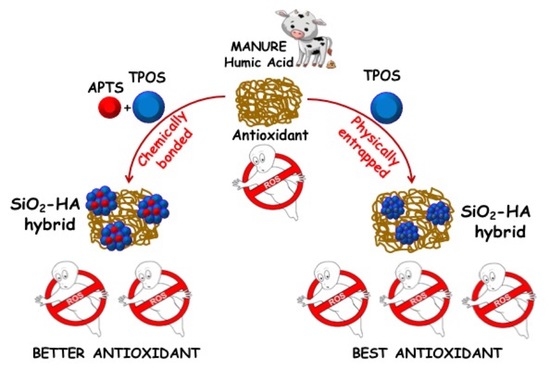
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Humic Acid Functionalized Silica Nanoparticles
2.3. Characterization Techniques
2.4. Ferrous Oxidation Xylenol Orange (FOX) Assay
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, C.; Nasrollahzadeh, M.; Selva, M.; Issaabadi, Z.; Luque, R. Waste-to-wealth: Biowaste valorization into valuable bio (nano) materials. Chem. Soc. Rev. 2019, 48, 4791–4822. [Google Scholar] [CrossRef]
- Glasing, J.; Champagne, P.; Cunningham, M.F. Current Opinion in Green and Sustainable Chemistry; Elsevier: Amsterdam, the Netherlands, 2016. [Google Scholar]
- Piccolo, A. The Supramolecular structure of humic substances. A novel understanding of humus chemistry and implications in soil Science. Adv. Agron. 2002, 75, 57–134. [Google Scholar]
- Yang, F.; Antonietti, M. The sleeping Giant: A Polymer View on Humic Matter in Synthesis and Applications. Prog. Polym. Sci. 2019, 100, 101182. [Google Scholar] [CrossRef]
- Piccolo, A.; Spaccini, R.; Drosos, M.; Vinci, G.; Cozzolino, V. The molecular composition of humus carbon: Recalcitrance and reactivity in soils. In The Future of Soil Carbon; Academic Press: Cambridge, MA, USA, 2018; pp. 87–124. [Google Scholar]
- Brezoiu, A.M.; Matei, C.; Deaconu, M.; Stanciuc, A.M.; Trifan, A.; Gaspar-Pintiliescu, A.; Berger, D. Polyphenols extract from grape pomace. Characterization and valorisation through encapsulation into mesoporous silica-type matrices. Food Chem. Toxicol. 2019, 133, 110787. [Google Scholar] [CrossRef] [PubMed]
- de Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar]
- Pukalchik, M.; Kydralieva, K.; Yakimenko, O.; Fedoseeva, E.; Terekhova, V. Outlining the potential role of humic products in modifying biological properties of the soil—A review. Front. Environ. Sci. 2019, 7, 80. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Yue, R.; Sun, X.F.; Song, C.; Wang, S.G. Enhanced removal of ciprofloxacin using humic acid modified hydrogel beads. J. Colloid Interface Sci. 2019, 543, 76–83. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Sangregorio, A.; Guigo, N.; van der Waal, J.C.; Sbirrazzuoli, N. Humins valorization: From well-defined properties to potential applications. AIP Conf. Proc. 2018, 1981, 020026. [Google Scholar]
- Tang, W.W.; Zeng, G.M.; Gong, J.L.; Liang, J.; Xu, P.; Zhang, C.; Huang, B.B. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci. Total Environ. 2014, 468, 1014–1027. [Google Scholar] [CrossRef]
- Milne, C.J.; Lapworth, D.J.; Gooddy, D.C.; Elgy, C.N.; Valsami-Jones, É. Role of humic acid in the stability of Ag nanoparticles in suboxic conditions. Environ. Sci. Technol. 2017, 51, 6063–6070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, N.C.; Urrutia, E.C.; Gertrudis, B.H.; Chaverri, J.P.; Mejía, G.B. Antioxidant activity of fulvic acid: A living matter-derived bioactive compound. J. Food Agric. Environ. 2011, 9, 123–127. [Google Scholar]
- Efimova, I.V.; Khil’ko, S.L.; Smirnova, O.V. Antioxidant activity of humic acids in radical-chain oxidation processes. Russ. J. Appl. Chem. 2012, 85, 1351–1354. [Google Scholar] [CrossRef]
- Klučáková, M.; Pekař, M. Solubility and dissociation of lignitic humic acids in water suspension. Colloids Surf. A Physicochem. Eng. Asp. 2005, 252, 157–163. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Z.; Lv, F.; Bie, X. Study on microencapsulation of curcumin pigments by spray drying. Eur. Food Res. Technol. 2009, 229, 391–396. [Google Scholar] [CrossRef]
- Deligiannakis, Y.; Sotiriou, G.A.; Pratsinis, S.E. Antioxidant and antiradical SiO2 nanoparticles covalently functionalized with gallic acid. ACS Appl. Mater. Interfaces 2012, 4, 6609–6617. [Google Scholar] [CrossRef]
- Liu, J.F.; Zhao, Z.S.; Jiang, G.B. Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef]
- Vitiello, G.; Zanfardino, A.; Tammaro, O.; Di Napoli, M.; Caso, M.F.; Pezzella, A.; Varcamonti, M.; Silvestri, B.; D’Errico, G.; Costantini, A.; et al. Bioinspired hybrid eumelanin–TiO2 antimicrobial nanostructures: The key role of organo–inorganic frameworks in tuning eumelanin’s biocide action mechanism through membrane interaction. RSC Adv. 2018, 8, 28275–28283. [Google Scholar] [CrossRef] [Green Version]
- Silvestri, B.; Vitiello, G.; Luciani, G.; Calcagno, V.; Costantini, A.; Gallo, M.; Parisi, S.; Paladino, S.; Iacomino, M.; D’Errico, G.; et al. Probing the eumelanin–silica interface in chemically engineered bulk hybrid nanoparticles for targeted subcellular antioxidant protection. ACS Appl. Mater. Interfaces 2017, 9, 37615–37622. [Google Scholar] [CrossRef]
- Vitiello, G.; Melone, P.; Silvestri, B.; Pezzella, A.; Di Donato, P.; D’Errico, G.; Di Napoli, M.; Zanfardino, A.; Varcamonti, M.; Luciani, G. Titanium based complexes with melanin precursors as a tool for directing melanogenic pathways. Pure Appl. Chem. 2019, 91, 1605–1616. [Google Scholar] [CrossRef]
- Vitiello, G.; Pezzella, A.; Calcagno, V.; Silvestri, B.; Raiola, L.; D’Errico, G.; Costantini, A.; Branda, F.; Luciani, G. 5,6-Dihydroxyindole-2-carboxylic acid–TiO2 charge transfer complexes in the radical polymerization of melanogenic precursor (s). J. Phys. Chem. C 2016, 120, 6262–6268. [Google Scholar] [CrossRef]
- Silvestri, B.; Pezzella, A.; Luciani, G.; Costantini, A.; Tescione, F.; Branda, F. Heparin conjugated silica nanoparticle synthesis. Mater. Sci. Eng. C 2012, 32, 2037–2041. [Google Scholar] [CrossRef]
- Ayyildiz, H.F. Evaluation of new silica—Based humic acid stationary phase for the separation of tocopherols in cold—Pressed oils by normal—Phase high—Performance liquid chromatography. J. Sep. Sci. 2015, 38, 813–820. [Google Scholar] [CrossRef]
- Prasetyo, E.; Toyoda, K. Sol–gel synthesis of a humic acid-silica gel composite material as low-cost adsorbent for thorium and uranium removal. J. Radioanal. Nucl. Chem. 2016, 310, 69–80. [Google Scholar] [CrossRef]
- Armon, R.; Zolkov, C.H.; Laor, Y. Entrapment of humic acid in a sol-gel matrix—A new tool for sorption studies. J. Sol-Gel Sci. Technol. 2000, 19, 95–100. [Google Scholar] [CrossRef]
- Laor, Y.; Zolkov, C.; Armon, R. Immobilizing humic acid in a sol-gel matrix: A new tool to study humic-contaminants sorption interactions. Environ. Sci. Technol. 2002, 36, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.Z.; Liu, P.; Su, X.C.; Liao, Y.H.; Lei, N.S.; Liang, Y.H.; Zhou, S.H.; Lin, W.S.; Chen, J.; Feng, Y.Q.; et al. Low-cost humic acid-bonded silica as an effective solid-phase extraction sorbent for convenient determination of aflatoxins in edible oils. Anal. Chim. Acta 2017, 970, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Prado, A.G.; Miranda, B.S.; Dias, J.A. Attachment of two distinct humic acids onto a silica gel surface. Colloids Surf. A Physicochem. Eng. Asp. 2004, 242, 137–143. [Google Scholar] [CrossRef]
- Sándor, M.; Nistor, C.L.; Szalontai, G.; Stoica, R.; Nicolae, C.A.; Alexandrescu, E.; Fazakas, J.; Oancea, F.; Donescu, D. Aminopropyl-silica hybrid particles as supports for humic acids immobilization. Materials 2016, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Kulikova, N.A.; Filippova, O.I.; Volikov, A.B.; Perminova, I.V. Slow nitrogen release from humic substances modified with aminoorganosilanes. J. Soil Sediments 2018, 18, 1400–1408. [Google Scholar] [CrossRef]
- Koopal, L.K.; Yang, Y.; Minnaard, A.J.; Theunissen, P.L.M.; Van Riemsdijk, W.H. Chemical immobilisation of humic acid on silica. Colloids Surf. A Physicochem. Eng. Asp. 1998, 141, 385–395. [Google Scholar] [CrossRef] [Green Version]
- Vinci, G.; Cozzolino, V.; Mazzei, P.; Monda, H.; Spaccini, R.; Piccolo, A. Effects of Bacillus amyloliquefaciens and organic and inorganic phosphate amendments on Maize plants as revealed by NMR and GC-MS based metabolomics. Plant Soil 2018, 429, 437–450. [Google Scholar] [CrossRef]
- Mazzei, P.; Vinale, F.; Woo, S.L.; Pascale, A.; Lorito, M.; Piccolo, A. Metabolomics by H-HRMAS-NMR of tomato plants treated with two secondary metabolites isolated from Trichoderma. J. Agric. Food Chem. 2016, 64, 3538–3545. [Google Scholar] [CrossRef] [Green Version]
- Nourooz-Zadeh, J.; Tajaddini-Sarmadi, J.; Wolff, S.P. Measurement of hydroperoxides in edible oils using the ferrous oxidation in xylenol orange assay. J. Agric. Food Chem. 1995, 43, 17–21. [Google Scholar] [CrossRef]
- Yang, L.; Lou, Z.; Han, X.; Liu, J.; Wang, Z.; Zhang, Y.; Wu, X.; Yuan, C.; Li, Y. Fabrication of a novel magnetic reconstituted bamboo with mildew resistance properties. Mater. Today Commun. 2020, 23, 101086. [Google Scholar] [CrossRef]
- Yuan, C.; Lou, Z.; Wang, W.; Yang, L.; Li, Y. Synthesis of Fe3C@C from Pyrolysis of Fe3O4-Lignin Clusters and Its Application for Quick and Sensitive Detection of PrPSc through a Sandwich SPR Detection Assay. Int. J. Mol. Sci. 2019, 20, 741. [Google Scholar] [CrossRef] [Green Version]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, D.; Ma, H.; Sun, Z.; Guan, J. Controllable preparation and formation mechanism of monodispersed silica particles with binary sizes. J. Colloid Interface Sci. 2012, 388, 40–46. [Google Scholar] [CrossRef]
- Masalov, V.M.; Sukhinina, N.S.; Kudrenko, E.A.; Emelchenko, G.A. Mechanism of formation and nanostructure of Stöber silica particles. Nanotechnology 2011, 22, 275718. [Google Scholar] [CrossRef]
- Passaro, J.; Russo, P.; Bifulco, A.; De Martino, M.T.; Granata, V.; Vitolo, B.; Iannace, G.; Vecchione, A.; Marulo, F.; Branda, F. Water Resistant Self-Extinguishing Low Frequency Soundproofing Polyvinylpyrrolidone Based Electrospun Blankets. Polymers 2019, 11, 1205. [Google Scholar] [CrossRef] [Green Version]
- Tadanaga, K.; Morita, K.; Mori, K.; Tatsumisago, M. Synthesis of monodispersed silica nanoparticles with high concentration by the Stöber process. J. Sol-Gel Sci. Technol. 2013, 68, 341–345. [Google Scholar] [CrossRef]
- Gezici, O.; Kara, H. Towards multimodal HPLC separations on humic acid-bonded aminopropyl silica: RPLC and HILIC behavior. Talanta 2011, 85, 1472–1482. [Google Scholar] [CrossRef]
- Branda, F.; Silvestri, B.; Costantini, A.; Luciani, G. Effect of exposure to growth media on size and surface charge of silica based Stöber nanoparticles: A DLS and ζ-potential study. J. Sol-Gel Sci. Technol. 2015, 73, 54–61. [Google Scholar] [CrossRef]
- Vialykh, E.A.; Salahub, D.R.; Achari, G.; Cook, R.L.; Langford, C.H. Emergent functional behaviour of humic substances perceived as complex labile aggregates of small organic molecules and oligomers. Environ. Chem. 2019, 16, 505–516. [Google Scholar] [CrossRef]
- Zhang, M.; Li, D.; Ye, Z.; Wang, S.; Xu, N.; Wang, F.; Liu, S.; Chen, J.; Gu, H. Effect of humic acid on the sedimentation and transport of nanoparticles silica in water-saturated porous media. J. Soils Sediments 2020, 20, 911–920. [Google Scholar] [CrossRef]
- Luciani, G.; Costantini, A.; Silvestri, B.; Tescione, F.; Branda, F.; Pezzella, A. Synthesis, structure and bioactivity of pHEMA/SiO2 hybrids derived through in situ sol–gel process. J. Sol-Gel Sci. Technol. 2008, 46, 166. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, Q.; Sun, L.; Tang, R.; Zhai, J. High efficient removal of mercury from aqueous solution by polyaniline/humic acid nanocomposite. J. Hazard. Mater. 2010, 175, 404–409. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.A.; Goulet, P.J.G.; Garrido, J.J. Characterization of the porous structure of different humic fractions. Colloids Surf. A Physicochem. Eng. Asp. 2005, 256, 129–135. [Google Scholar] [CrossRef]
- Luo, D.; Yu, Q.W.; Yin, H.R.; Feng, Y.Q. Humic acid-bonded silica as a novel sorbent for solid-phase extraction of benzo [a] pyrene in edible oils. Anal. Chim. Acta 2007, 588, 261–267. [Google Scholar] [CrossRef]
- Amir, S.; Jouraiphy, A.; Meddich, A.; El Gharous, M.; Winterton, P.; Hafidi, M. Structural study of humic acids during composting of activated sludge-green waste: Elemental analysis, FTIR and 13C NMR. J. Hazard. Mater. 2010, 177, 524–529. [Google Scholar] [CrossRef]
- Yu, X.; Yu, X.; Wu, S.; Liu, B.; Liu, H.; Guan, J.; Kan, Q. The effect of the distance between acidic site and basic site immobilized on mesoporous solid on the activity in catalyzing aldol condensation. J. Solid State Chem. 2011, 184, 289–295. [Google Scholar] [CrossRef]
- Nhlapo, N.S.; Focke, W.W.; Vuorinen, E. TGA–FTIR study of the vapors released by triethylamine–acetic acid mixtures. Thermochim. Acta 2012, 546, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Nuzzo, A.; Piccolo, A. Enhanced catechol oxidation by heterogeneous biomimetic catalysts immobilized on clay minerals. J. Mol. Catal. A Chem. 2013, 371, 8–14. [Google Scholar] [CrossRef]
- Califano, V.; Sannino, F.; Costantini, A.; Avossa, J.; Cimino, S.; Aronne, A. Wrinkled silica nanoparticles: Efficient matrix for β-glucosidase immobilization. J. Phys. Chem. C 2018, 122, 8373–8379. [Google Scholar] [CrossRef]
- Sannino, F.; Costantini, A.; Ruffo, F.; Aronne, A.; Venezia, V.; Califano, V. Covalent Immobilization of β-Glucosidase into Mesoporous Silica Nanoparticles from Anhydrous Acetone Enhances Its Catalytic Performance. Nanomaterials 2020, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Norcino, L.B.; de Oliveira, J.E.; Moreira, F.K.; Marconcini, J.M.; Mattoso, L.H. Rheological and thermo-mechanical evaluation of bio-based chitosan/pectin blends with tunable ionic cross-linking. Int. J. Biol. Macromol. 2018, 118, 1817–1823. [Google Scholar] [CrossRef]
- Yang, J.; Stuart, M.A.C.; Kamperman, M. Jack of all trades: Versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 2014, 43, 8271–8298. [Google Scholar] [CrossRef]
- Panzella, L.; D’Errico, G.; Vitiello, G.; Perfetti, M.; Alfieri, M.L.; Napolitano, A.; D’Ischia, M.; D’Errico, G.; D’Ischia, M. Disentangling structure-dependent antioxidant mechanisms in phenolic polymers by multiparametric EPR analysis. Chem. Commun. 2018, 54, 9426–9429. [Google Scholar] [CrossRef]
- Buszman, E.; Pilawa, B.; Zdybel, M.; Wilczyński, S.; Gondzik, A.; Witoszyńska, T.; Wilczokc, T. EPR examination of Zn2+ and Cu2+ binding by pigmented soil fungi Cladosporium cladosporioides. Sci. Total Environ. 2006, 363, 195–205. [Google Scholar] [CrossRef]











| Peak Position (cm−1) | Vibrational Mode |
|---|---|
| ~1100 | Si–O–Si stretching |
| ~950 | Si–O–Si bond between two adjacent tetrahedra |
| ~800 | Si–O–Si stretching |
| ~470 | Si–O–Si bending |
| Range (ppm) | Attribution | HA |
|---|---|---|
| 0–45 | Alkyl C | 19.52 |
| 45–60 | Methoxyl C | 12.78 |
| 60–110 | O-Alkyl C | 55.00 |
| 110–145 | Aryl C | 12.08 |
| 145–160 | O-aryl C | 4.47 |
| 160–190 | Carboxyl | 8.23 |
| Sample | g-Factor (±0.004) | ΔB (±0.2 G) | Spin × g−1 × 1017 (err. ≤ 10%) |
|---|---|---|---|
| HA | 2.0035 | 6.5 ± 0.2 | 2.10 |
| SiO2/HA_p | 2.0034 | 6.2 ± 0.2 | 0.32 |
| SiO2/HA_c | 2.0037 | 7.7 ± 0.2 | 5.80 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pota, G.; Venezia, V.; Vitiello, G.; Di Donato, P.; Mollo, V.; Costantini, A.; Avossa, J.; Nuzzo, A.; Piccolo, A.; Silvestri, B.; et al. Tuning Functional Behavior of Humic Acids through Interactions with Stöber Silica Nanoparticles. Polymers 2020, 12, 982. https://doi.org/10.3390/polym12040982
Pota G, Venezia V, Vitiello G, Di Donato P, Mollo V, Costantini A, Avossa J, Nuzzo A, Piccolo A, Silvestri B, et al. Tuning Functional Behavior of Humic Acids through Interactions with Stöber Silica Nanoparticles. Polymers. 2020; 12(4):982. https://doi.org/10.3390/polym12040982
Chicago/Turabian StylePota, Giulio, Virginia Venezia, Giuseppe Vitiello, Paola Di Donato, Valentina Mollo, Aniello Costantini, Joshua Avossa, Assunta Nuzzo, Alessandro Piccolo, Brigida Silvestri, and et al. 2020. "Tuning Functional Behavior of Humic Acids through Interactions with Stöber Silica Nanoparticles" Polymers 12, no. 4: 982. https://doi.org/10.3390/polym12040982
APA StylePota, G., Venezia, V., Vitiello, G., Di Donato, P., Mollo, V., Costantini, A., Avossa, J., Nuzzo, A., Piccolo, A., Silvestri, B., & Luciani, G. (2020). Tuning Functional Behavior of Humic Acids through Interactions with Stöber Silica Nanoparticles. Polymers, 12(4), 982. https://doi.org/10.3390/polym12040982