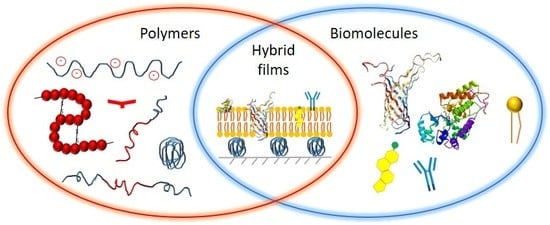
Recent Advances in Hybrid Biomimetic Polymer-Based Films: from Assembly to Applications
Abstract
:1. Introduction
2. Planar Hybrid Systems Based on Polyelectrolytes
2.1. Assembly of Hybrid PE-Based Membranes
2.2. Properties and Applications of Biomimetic Hybrid Membranes Based on PE
2.2.1. PE-Natural Polymer Hybrid Films
2.2.2. PE-Nucleic Acid Hybrid Layers
2.2.3. PE-Protein Hybrid Multilayers
2.2.4. PE-Enzyme Conjugated Planar Systems
3. Hybrid Films Based on Polymer Brushes
3.1. Polymer Films Obtained via the Grafting “from” Approach
3.2. Polymer Films Obtained via the Grafting “to” Approach
3.3. Properties and Applications of Hybrid Films Based on Polymer Brushes
4. Polymer-Lipid Tethered and Cushioned Composite Films
4.1. Polymer-Tethered Lipid Bilayer Membranes
4.2. Polymer-Cushioned Lipid Bilayer Membranes
4.3. Properties and Applications of Polymer-Lipid Composite Films
5. Hybrid Polymer-Lipid Supported Membranes
5.1. Assembly of Hybrid Membranes Based on Polymers and Lipids
5.2. Properties and Applications of Polymer-Lipid Membranes
6. Conclusions
List of Abbreviations
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Avsar, S.Y.; Kyropoulou, M.; di Leone, S.; Schoenenberger, C.-A.; Meier, W.P.; Palivan, C.G. Biomolecules Turn Self-Assembling Amphiphilic Block Co-Polymer Platforms into Biomimetic Interfaces. Front. Chem. 2019, 6, 645. [Google Scholar] [CrossRef] [PubMed]
- Eeman, M.; Deleu, M. From Biological Membranes to Biomimetic Model Membranes. Biotechnol. Agron. Société Et Environ. 2010, 14, 719–736. [Google Scholar]
- Carmona-Ribeiro, A.M. Biomimetic Nanomaterials from the Assembly of Polymers, Lipids and Surfactants. Surfactants Deterg. 2019. [Google Scholar]
- Watson, H. Biological Membranes. Essays Biochem. 2015, 59, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Kita-Tokarczyk, K.; Itel, F.; Grzelakowski, M.; Egli, S.; Rossbach, P.; Meier, W. Monolayer Interactions between Lipids and Amphiphilic Block Copolymers. Langmuir 2009, 25, 9847–9856. [Google Scholar] [CrossRef]
- Kowal, J.; Wu, D.; Mikhalevich, V.; Palivan, C.G.; Meier, W. Hybrid Polymer–Lipid Films as Platforms for Directed Membrane Protein Insertion. Langmuir 2015, 31, 4868–4877. [Google Scholar] [CrossRef]
- Discher, D.E.; Adi, E. Polymer Vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Contreras, F.; Ernst, A.M.; Wieland, F.; Brügger, B. Specificity of Intramembrane Protein–Lipid Interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a004705. [Google Scholar] [CrossRef] [Green Version]
- Criado, M.; Rebollar, E.; Nogales, A.; Ezquerra, T.A.; Boulmedais, F.; Mijangos, C.; Hernández, R. Quantitative nanomechanical properties of multilayer films made of polysaccharides through spray assisted layer-by-layer assembly. Biomacromolecules 2017, 18, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Criado-Gonzalez, M.; Corbella, L.; Senger, B.; Boulmedais, F.; Hernández, R. Photoresponsive Nanometer-Scale Iron Alginate Hydrogels: A Study of Gel–Sol Transition Using a Quartz Crystal Microbalance. Langmuir 2019, 35, 11397–11405. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Álvarez, L.; Ruiz-Rubio, L.; Lizundia, E.; Hernáez, E.; León, L.M.; Vilas-Vilela, J.L. Active Release Coating of Multilayer Assembled Branched and Ionic Β-Cyclodextrins onto Poly (Ethylene Terephthalate). Carbohydr. Polym. 2017, 174, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Morshed, M.N.; Behary, N.; Bouazizi, N.; Guan, J.; Chen, G.; Nierstrasz, V. Surface Modification of Polyester Fabric Using Plasma-Dendrimer for Robust Immobilization of Glucose Oxidase Enzyme. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Grunlan, J.C.; Choi, J.K.; Lin, A. Antimicrobial Behavior of Polyelectrolyte Multilayer Films Containing Cetrimide and Silver. Biomacromolecules 2005, 6, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Vörös, J. An Aqueous-Based Surface Modification of Poly (Dimethylsiloxane) with Poly (Ethylene Glycol) to Prevent Biofouling. Langmuir 2005, 21, 11957–11962. [Google Scholar] [CrossRef]
- Stoenescu, R.; Alexandra, G.; Wolfgang, M. Asymmetric Abc-Triblock Copolymer Membranes Induce a Directed Insertion of Membrane Proteins. Macromol. Biosci. 2004, 4, 930–935. [Google Scholar] [CrossRef]
- Leiske, D.L.; Meckes, B.; Miller, C.E.; Wu, C.; Walker, T.W.; Lin, B.; Meron, M.; Ketelson, H.A.; Toney, M.F.; Fuller, G.G. Insertion Mechanism of a Poly (Ethylene Oxide)-Poly (Butylene Oxide) Block Copolymer into a Dppc Monolayer. Langmuir 2011, 27, 11444–11450. [Google Scholar] [CrossRef]
- Caruso, F.; Furlong, D.N.; Ariga, K.; Ichinose, I.; Kunitake, T. Characterization of Polyelectrolyte−Protein Multilayer Films by Atomic Force Microscopy, Scanning Electron Microscopy.; Fourier Transform Infrared Reflection− Absorption Spectroscopy. Langmuir 1998, 14, 4559–4565. [Google Scholar] [CrossRef]
- Li, Q.; Quinn, J.F.; Wang, Y.; Caruso, F. Preparation of Nanoporous Polyelectrolyte Multilayer Films Via Nanoparticle Templating. Chem. Mater. 2006, 18, 5480–5485. [Google Scholar] [CrossRef]
- Tang, D.; Xia, B.; Zhang, Y. Direct Electrochemistry and Electrocatalysis of Hemoglobin in a Multilayer {Nanogold/Pdda} N Inorganic–Organic Hybrid Film. Microchim. Acta 2008, 160, 367–374. [Google Scholar] [CrossRef]
- Bi, S.; Zhou, H.; Zhang, S. Multilayers Enzyme-Coated Carbon Nanotubes as Biolabel for Ultrasensitive Chemiluminescence Immunoassay of Cancer Biomarker. Biosens. Bioelectron. 2009, 24, 2961–2966. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, G.; Wang, J. Biosensors Fabricated through Electrostatic Assembly of Enzymes/Polyelectrolyte Hybrid Layers on Carbon Nanotubes. In Proceedings of the Nanotech 2006 Technical, Boston, MA, USA, 7–11 May 2006. [Google Scholar]
- Wang, J.; Liu, G.; Lin, Y. Amperometric Choline Biosensor Fabricated through Electrostatic Assembly of Bienzyme/Polyelectrolyte Hybrid Layers on Carbon Nanotubes. Analyst 2006, 131, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Liu, L.; Wang, S. Fluorescent Conjugated Polymer-Based Fret Technique for Detection of DNA Methylation of Cancer Cells. Nat. Protoc. 2010, 5, 1255. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of Nanoparticles in Electrochemical Sensors and Biosensors. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2006, 18, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Motorina, A.O.; Tananaiko, I.; Kozytska, V.; Raks, R.; Badía, M.E.; Díaz-García, V.; Zaitsev, N. Hybrid Silica–Polyelectrolyte Films as Optical Sensing Materials for Tetracycline Antibiotics. Sens. Actuators B Chem. 2014, 200, 198–205. [Google Scholar] [CrossRef]
- Hillebrandt, H.; Wiegand, G.; Tanaka, M.; Sackmann, E. High Electric Resistance Polymer/Lipid Composite Films on Indium− Tin− Oxide Electrodes. Langmuir 1999, 15, 8451–8459. [Google Scholar] [CrossRef]
- Hillebrandt, H.; Tanaka, M.; Sackmann, E. A Novel Membrane Charge Sensor: Sensitive Detection of Surface Charge at Polymer/Lipid Composite Films on Indium Tin Oxide Electrodes. J. Phys. Chem. B 2002, 106, 477–486. [Google Scholar] [CrossRef]
- Harada, Y.; Noda, J.; Yatabe, R.; Ikezaki, H.; Toko, K. Research on the Changes to the Lipid/Polymer Membrane Used in the Acidic Bitterness Sensor Caused by Preconditioning. Sensors 2016, 16, 230. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Habara, M.; Ikezaki, H.; Toko, K. Study of Surface-Modified Lipid/Polymer Membranes for Detecting Sweet Taste Substances. In Proceedings of the 2008 3rd International Conference on Sensing Technology, Tainan, Taiwan, Province of China; 2008. [Google Scholar]
- Nakatani, F.; Ienaga, T.; Wu, X.; Tahara, Y.; Ikezaki, H.; Sano, H.; Muto, Y.; Kaneda, Y.; Toko, K. Development of a Sensor with a Lipid/Polymer Membrane Comprising Na+ Ionophores to Evaluate the Saltiness Enhancement Effect. Sensors 2019, 19, 5251. [Google Scholar] [CrossRef] [Green Version]
- Larraneta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle Arrays as Transdermal and Intradermal Drug Delivery Systems: Materials Science, Manufacture and Commercial Development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.W.; Lee, M.S.; Kim, K.R.; Lee, J.E.; Lee, K.; Park, J.S.; Matsumoto, Y.; Jo, D.G.; Lee, H.; Lee, D.S.; et al. Polyplex-Releasing Microneedles for Enhanced Cutaneous Delivery of DNA Vaccine. J. Control. Release 2014, 179, 11–17. [Google Scholar] [CrossRef]
- Liao, J.; Lee, J.-C.; Lin, C.-K.; Wei, K.-C.; Chen, P.-Y.; Yang, H.-W. Self-Assembly DNA Polyplex Vaccine inside Dissolving Microneedles for High-Potency Intradermal Vaccination. Theranostics 2017, 7, 2593. [Google Scholar] [CrossRef]
- DeMuth, P.C.; Su, X.; Samuel, R.E.; Hammond, P.T.; Irvine, D.J. Nano-Layered Microneedles for Transcutaneous Delivery of Polymer Nanoparticles and Plasmid DNA. Adv. Mater. 2010, 22, 4851–4856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Intra, J.; Salem, A.K. Comparative Study of Poly (Lactic-Co-Glycolic Acid)-Poly Ethyleneimine-Plasmid DNA Microparticles Prepared Using Double Emulsion Methods. J. Microencapsul. 2008, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, C.; Zinchenko, A. Small DNA Additives to Polyelectrolyte Multilayers Promote Formation of Ultrafine Gold Nanoparticles with Enhanced Catalytic Activity. Colloid Polym. Sci. 2019, 297, 363–369. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Kim, N.W.; Thambi, T.; Phan, V.H.G.; Lee, M.S.; Yin, Y.; Jeong, J.H.; Lee, D.S. Microneedle Arrays Coated with Charge Reversal Ph-Sensitive Copolymers Improve Antigen Presenting Cells-Homing DNA Vaccine Delivery and Immune Responses. J. Control. Release 2018, 269, 225–234. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Yin, Y.; Thambi, T.; Nguyen, T.L.; Phan, V.H.G.; Lee, M.S.; Lee, J.E.; Kim, J.; Jeong, J.H.; Lee, D.S. Smart Vaccine Delivery Based on Microneedle Arrays Decorated with Ultra-Ph-Responsive Copolymers for Cancer Immunotherapy. Biomaterials 2018, 185, 13–24. [Google Scholar] [CrossRef]
- Handwerger, R.G.; Diamond, S.L. Biotinylated Photocleavable Polyethylenimine: Capture and Triggered Release of Nucleic Acids from Solid Supports. Bioconj. Chem. 2007, 18, 717–723. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, Q.; Zhang, P.; Zhao, X.; Wang, Y. Cutaneous Microenvironment Responsive Microneedle Patch for Rapid Gene Release to Treat Subdermal Tumor. J. Control. Release 2019, 314, 72–80. [Google Scholar] [CrossRef]
- Baker, A.; Saltik, M.; Lehrmann, H.; Killisch, I.; Mautner, V.; Lamm, G.; Christofori, G.; Cotten, M. Polyethylenimine (Pei) Is a Simple, Inexpensive and Effective Reagent for Condensing and Linking Plasmid DNA to Adenovirus for Gene Delivery. Gene Ther. 1997, 4, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Green, M.D.; Foster, A.A.; Greco, C.T.; Roy, R.; Lehr, R.M.; Thomas, H.E., III; Sullivan, M.O. Catch and Release: Photocleavable Cationic Diblock Copolymers as a Potential Platform for Nucleic Acid Delivery. Polym. Chem. 2014. [Google Scholar] [CrossRef] [Green Version]
- Tuteja, M.; Kang, M.; Leal, C.; Centrone, A. Nanoscale Partitioning of Paclitaxel in Hybrid Lipid–Polymer Membranes. Analyst 2018, 143, 3808–3813. [Google Scholar] [CrossRef] [PubMed]
- Tamm, L.K.; McConnell, H.M. Supported Phospholipid Bilayers. Biophys. J. 1985, 47, 105. [Google Scholar] [CrossRef] [Green Version]
- Perez, T.D.; Nelson, W.J.; Boxer, S.G.; Kam, L. E-Cadherin Tethered to Micropatterned Supported Lipid Bilayers as a Model for Cell Adhesion. Langmuir 2005, 21, 11963–11968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagoda, A.; Ketikidis, P.; Zinn, M.; Meier, W.; Kita-Tokarczyk, K. Interactions of Biodegradable Poly ([R]-3-Hydroxy-10-Undecenoate) with 1, 2-Dioleoyl-Sn-Glycero-3-Phosphocholine Lipid: A Monolayer Study. Langmuir 2011, 27, 10878–10885. [Google Scholar] [CrossRef]
- Chemin, M.; Brun, P.; Lecommandoux, S.; Sandre, O.; le Meins, J. Hybrid Polymer/Lipid Vesicles: Fine Control of the Lipid and Polymer Distribution in the Binary Membrane. Soft Matter 2012, 8, 2867–2874. [Google Scholar] [CrossRef] [Green Version]
- Kwon, C.H.; Ko, Y.; Shin, D.; Kwon, M.; Park, J.; Bae, W.K.; Lee, S.W.; Cho, J. High-Power Hybrid Biofuel Cells Using Layer-by-Layer Assembled Glucose Oxidase-Coated Metallic Cotton Fibers. Nat. Commun. 2018, 9, 4479. [Google Scholar] [CrossRef] [Green Version]
- Zinchenko, A.; Nagahama, C.; Murata, S. Gold Nanoparticles in DNA-Based Multilayer Films: Synthesis, Size Control.; Influence of the Multilayer Structure on Catalytic Properties. ChemNanoMat 2016, 2, 125–132. [Google Scholar] [CrossRef]
- Liu, G.; Shao, Y.; Peng, J.; Dai, W.; Liu, L.; Xu, S.; Wu, F.; Wu, X. Highly Thymine-Dependent Formation of Fluorescent Copper Nanoparticles Templated by ss-DNA. Nanotechnology 2013, 24, 345502. [Google Scholar] [CrossRef]
- Ocsoy, I.; Gulbakan, B.; Chen, T.; Zhu, G.; Chen, Z.; Sari, M.M.; Peng, L.; Xiong, X.; Fang, X.; Tan, W. DNA-Guided Metal-Nanoparticle Formation on Graphene Oxide Surface. Adv. Mater. 2013, 25, 2319–2325. [Google Scholar] [CrossRef]
- Perrino, C.; Lee, S.; Spencer, N.D. End-Grafted Sugar Chains as Aqueous Lubricant Additives: Synthesis and Macrotribological Tests of Poly (L-Lysine)-Graft-Dextran (Pll-G-Dex) Copolymers. Tribol. Lett. 2009, 33, 83–96. [Google Scholar] [CrossRef]
- Perrino, C.; Lee, S.; Choi, S.W.; Maruyama, A.; Spencer, N.D. A Biomimetic Alternative to Poly (Ethylene Glycol) as an Antifouling Coating: Resistance to Nonspecific Protein Adsorption of Poly (L-Lysine)-Graft-Dextran. Langmuir 2008, 24, 8850–8856. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, G.; Meng, Q.; Ding, C.; Jiang, H.; Fang, Y. A biomimetic nano hybrid coating based on the lotus effect and its anti-biofouling behaviors. Appl. Surf. Sci. 2014, 315, 407–414. [Google Scholar] [CrossRef]
- Koetz, J.; Kosmella, S. Polyelectrolytes and Nanoparticles; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- Radeva, T. Physical Chemistry of Polyelectrolytes; CRC Press: Boca Raton, FL, USA, 2001; Volume 99. [Google Scholar]
- Demann, E.P.P. Zwitterion Silane-Modified Polymer Latexes. In Polyelectrolytes and their applications; Rembaum, A., Selegny, E., Eds.; Springer: Berlin, Germany, 2012; Volume 2, p. 119. [Google Scholar]
- Thompson, C.; Cheng, W.P. Chemically Modified Polyelectrolytes for Intestinal Peptide and Protein Delivery. In Peptide and Protein Delivery; Elsevier: Amsterdam, The Netherlands, 2011; pp. 123–164. [Google Scholar]
- Abdallah, B.G.; Ros, A. Surface Coatings for Microfluidic-Based Biomedical Devices. In Microfluidic Devices for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2013; pp. 63–99. [Google Scholar]
- Sonia, T.A.; Sharma, C.P. 5-Lipids and Inorganic Nanoparticles in Oral Insulin Delivery. In Oral Delivery of Insulin; Woodhead Publishing: Waltham, MA, USA, 2014; pp. 219–256. [Google Scholar]
- Lankalapalli, S.; Kolapalli, V.R.M. Polyelectrolyte Complexes: A Review of Their Applicability in Drug Delivery Technology. Indian J. Pharm. Sci. 2009, 71, 481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.K.; Singh, V.K.; Singh, M. Zwitterionic Polyelectrolytes: A Review. e-Polymers 2007, 7. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Q.; Yuan, J. Porous Polyelectrolytes: The Interplay of Charge and Pores for New Functionalities. Angew. Chem. Int. Ed. 2018, 57, 6754–6773. [Google Scholar] [CrossRef]
- Stuart, M.C.; Lyklema, J. Fundamentals of Interface and Colloid Science: Soft Colloids; Elsevier: Amsterdam, The Netherlands, 2005; Volume 5. [Google Scholar]
- McKinney, D.; Sigmund, W. Handbook of Advanced Ceramics, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Tieke, B.; Toutianoush, A. Electrostatic Layer-by-Layer Fabrication of Ultrathin Separation Membranes. In Bottom-Up, Nanofabrication; Ariga, K., Nalwa, H.S., Eds.; Organized Films, American Scientific Publications: Stevenson Ranch, CA, USA, 2009; Volume 5, p. 202. [Google Scholar]
- Bruening, M.; Daniel, L.; Sulliva, M. Enhancing the Ion-Transport Selectivity of Multilayer Polyelectrolyte Membranes. Chem. A Eur. J. 2002, 8, 3832–3837. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Decher, G.; Schlenoff, J.B. Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Li, Y.; Wang, X.; Sun, J. Layer-by-Layer Assembly for Rapid Fabrication of Thick Polymeric Films. Chem. Soc. Rev. 2012, 41, 5998–6009. [Google Scholar] [CrossRef]
- Park, K.; Choi, D.; Hong, J. Nanostructured Polymer Thin Films Fabricated with Brush-Based Layer-by-Layer Self-Assembly for Site-Selective Construction and Drug Release. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Cheng, W.; Wang, T.; Wang, Y.; Wang, E.; Dong, S. Fabrication, Characterization.; Application in Sers of Self-Assembled Polyelectrolyte−Gold Nanorod Multilayered Films. J. Phys. Chem. B 2005, 109, 19385–19389. [Google Scholar] [CrossRef]
- Chen, L.; Lu, G. Direct Electrochemistry and Electrocatalysis of Hybrid Film Assembled by Polyelectrolyte–Surfactant Polymer, Carbon Nanotubes and Hemoglobin. J. Electroanal. Chem. 2006, 597, 51–59. [Google Scholar] [CrossRef]
- Frau, A.F.; Estillore, N.C.; Fulghum, T.M.; Advincula, R.C. Intercalative Poly(Carbazole) Precursor Electropolymerization within Hybrid Nanostructured Titanium Oxide Ultrathin Films. ACS Appl. Mater. Interfaces 2010, 2, 3726–3737. [Google Scholar] [CrossRef] [PubMed]
- Maerten, C.; Lopez, L.; Lupattelli, P.; Rydzek, G.; Pronkin, S.; Schaaf, P.; Jierry, L.; Boulmedais, F. Electrotriggered Confined Self-Assembly of Metal–Polyphenol Nanocoatings Using a Morphogenic Approach. Chem. Mater. 2017, 29, 9668–9679. [Google Scholar] [CrossRef] [Green Version]
- Yameen, B.; Kaltbeitzel, A.; Glasser, G.; Langner, A.; Muller, F.; Gösele, U.; Knoll, W.; Azzaroni, O. Hybrid Polymer−Silicon Proton Conducting Membranes Via a Pore-Filling Surface-Initiated Polymerization Approach. ACS Appl. Mater. Interfaces 2009, 2, 279–287. [Google Scholar] [CrossRef]
- Schneider, A.; Francius, G.; Obeid, R.; Schwinté, P.; Hemmerlé, J.; Frisch, B.; Schaaf, P.; Voegel, J.-C.; Senger, B.; Picart, C. Polyelectrolyte Multilayers with a Tunable Young’s Modulus: Influence of Film Stiffness on Cell Adhesion. Langmuir 2006, 22, 1193–1200. [Google Scholar] [CrossRef]
- Richert, L.; Boulmedais, F.; Lavalle, P.; Mutterer, J.; Ferreux, E.; Decher, G.; Schaaf, P.; Voegel, J.; Picart, C. Improvement of Stability and Cell Adhesion Properties of Polyelectrolyte Multilayer Films by Chemical Cross-Linking. Biomacromolecules 2004, 5, 284–294. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Ji, S.; Zhang, G.; Li, J.; Wang, L. Self-Assembly of Graphene Oxide and Polyelectrolyte Complex Nanohybrid Membranes for Nanofiltration and Pervaporation. Chem. Eng. J. 2012, 213, 318–329. [Google Scholar] [CrossRef]
- Lee, C.-W.; Park, H.-S.; Kim, J.-G.; Choi, B.-K.; Joo, S.-W.; Gong, M.-S. Polymeric Humidity Sensor Using Organic/Inorganic Hybrid Polyelectrolytes. Sens. Actuators B Chem. 2005, 109, 315–322. [Google Scholar] [CrossRef]
- Lee, C.; Park, H.-S.; Kim, J.-G.; Gong, M.-S. Humidity Sensitivity of Hybrid Polyelectrolytes Prepared by the Sol-Gel Process. Macromol. Res. 2005, 13, 96–101. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Shahi, V.K. Functionalized Organic−Inorganic Nanostructured N-P-Carboxy Benzyl Chitosan−Silica−Pva Hybrid Polyelectrolyte Complex as Proton Exchange Membrane for Dmfc Applications. J. Phys. Chem. B 2008, 112, 15678–15690. [Google Scholar] [CrossRef]
- Su, P.-G.; Sun, Y.-L.; Wang, C.-S.; Lin, C.-C. Humidity Sensing and Electrical Properties of Hybrid Films Prepared from [3-(Methacrylamino)Propyl] Trimethyl Ammonium Chloride, Aqueous Monodispersed Colloidal Silica and Methyl Methacrylate. Sens. Actuators B Chem. 2006, 119, 483–489. [Google Scholar] [CrossRef]
- Martínez, Y.; Jaime, R.; Mehrdad, Y.-P.; Helmut, C. Transparent Semiconductor–Polymer Hybrid Films with Tunable Optical Properties. J. Mater. Chem. 2007, 17, 1094–1101. [Google Scholar]
- Zhou, Y.; Ma, R.; Ebina, Y.; Takada, K.; Sasaki, T. Multilayer Hybrid Films of Titania Semiconductor Nanosheet and Silver Metal Fabricated Via Layer-by-Layer Self-Assembly and Subsequent Uv Irradiation. Chem. Mater. 2006, 18, 1235–1239. [Google Scholar] [CrossRef]
- Park, M.; Lim, T.; Jeon, Y.-M.; Kim, J.-G.; Joo, S.-W.; Gong, M.-S. Humidity Sensitive Properties of Copoly(Teamps/Vp)/Silver Nanocomposite Films. Sens. Actuators B Chem. 2008, 133, 166–173. [Google Scholar] [CrossRef]
- Lv, X.; Li, Y.; Hong, L.; Luo, D.; Yang, M. A Highly Water-Resistive Humidity Sensor Based on Silicon-Containing Polyelectrolytes Prepared by One-Pot Method. Sens. Actuators B Chem. 2007, 124, 347–351. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, L.; Liu, F.; Ji, M.; Tang, W.; Pang, M.; Sun, J. Exponential Growth of Layer-by-Layer Assembled Coatings with Well-Dispersed Ultrafine Nanofillers: A Facile Route to Scratch-Resistant and Transparent Hybrid Coatings. J. Mater. Chem. 2010, 20, 7721–7727. [Google Scholar] [CrossRef]
- Yao, H.-B.; Wu, L.-H.; Cui, C.-H.; Fang, H.-Y.; Yu, S.-H. Direct Fabrication of Photoconductive Patterns on Lbl Assembled Graphene Oxide/Pdda/Titania Hybrid Films by Photothermal and Photocatalytic Reduction. J. Mater. Chem. 2010, 20, 5190–5195. [Google Scholar] [CrossRef]
- Lee, D.; Choi, M.-C.; Ha, C.-S. Polynorbornene Dicarboximide/Amine Functionalized Graphene Hybrids for Potential Oxygen Barrier Films. J. Polym. Sci. Part. A Polym. Chem. 2012, 50, 1611–1621. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Mikhailovsky, A.; Bazan, G.C. Conjugated Polyelectrolyte–Metal Nanoparticle Platforms for Optically Amplified DNA Detection. Adv. Mater. 2010, 22, 656–659. [Google Scholar] [CrossRef]
- Dementiev, A.A.; Baikov, A.A.; Ptushenko, V.V.; Khomutov, G.B.; Tikhonov, A.N. Biological and Polymeric Self-Assembled Hybrid Systems: Structure and Properties of Thylakoid/Polyelectrolyte Complexes. Biochim. Et Biophys. Acta Biomembr. 2005, 1712, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Janáky, C.; Visy, C. Conducting Polymer-Based Hybrid Assemblies for Electrochemical Sensing: A Materials Science Perspective. Anal. Bioanal. Chem. 2013, 405, 3489–3511. [Google Scholar] [CrossRef] [PubMed]
- Uto, K.; Yamamoto, K.; Kishimoto, N.; Muraoka, M.; Aoyagi, T.; Yamashita, I. Electrostatic Adsorption of Ferritin, Proteins and Nanoparticle Conjugate onto the Surface of Polyelectrolyte Multilayers. J. Mater. Chem. 2008, 18, 3876–3884. [Google Scholar] [CrossRef]
- Caruso, F.; Trau, D.; Möhwald, H.; Renneberg, R. Enzyme Encapsulation in Layer-by-Layer Engineered Polymer Multilayer Capsules. Langmuir 2000, 16, 1485–1488. [Google Scholar] [CrossRef]
- Diaspro, A.; Silvano, D.; Krol, S.; Cavalleri, O.; Gliozzi, A. Single Living Cell Encapsulation in Nano-Organized Polyelectrolyte Shells. Langmuir 2002, 18, 5047–5050. [Google Scholar] [CrossRef]
- Zamarreño, C.; Javier Bravo, R.J.; Goicoechea, I.R.; Matias, F.; Arregui, J. Response time enhancement of pH sensing films by means of hydrophilic nanostructured coatings. Sens. Actuators B Chem. 2007, 128, 138–144. [Google Scholar] [CrossRef]
- Kharalampieva, E.; Sukhishvili, S.A. Release of a Dye from Hydrogen-Bonded and Electrostatically Assembled Polymer Films Triggered by Adsorption of a Polyelectrolyte. Langmuir 2004, 20, 9677–9685. [Google Scholar] [CrossRef]
- Wong, J.E.; Gaharwar, A.K.; Müller-Schulte, D.; Bahadur, D.; Richtering, W. Layer-by-layer assembly of a magnetic nanoparticle shell on a thermoresponsive microgel core. J. Magn. Magn. Mater. 2007, 311, 219–223. [Google Scholar] [CrossRef]
- Milkova, V. Polyelectrolyte/Nanoparticle Hybrid Films on Anisometric Colloids Studied by Electro-optics. Colloids Surf. A Physicochem. Eng. Asp. 2014, 455, 156–163. [Google Scholar] [CrossRef]
- Peng, C.; Thio, Y.S.; Gerhardt, R.A. Effect of Precursor-Layer Surface Charge on the Layer-by-Layer Assembly of Polyelectrolyte/Nanoparticle Multilayers. Langmuir 2012, 28, 84–91. [Google Scholar] [CrossRef]
- Peng, C.; Thio, Y.S.; Gerhardt, R.A.; Ambaye, H.; Lauter, V. Ph-Promoted Exponential Layer-by-Layer Assembly of Bicomponent Polyelectrolyte/Nanoparticle Multilayers. Chem. Mater. 2011, 23, 4548–4556. [Google Scholar] [CrossRef]
- Chen, T.; Somasundaran, P. Preparation of Novel Core-Shell Nanocomposite Particles by Controlled Polymer Bridging. J. Am. Ceram. Soc. 1998, 81, 140–144. [Google Scholar] [CrossRef]
- Jisr, R.M.; Rmaile, H.H.; Schlenoff, J.B. Hydrophobic and Ultrahydrophobic Multilayer Thin Films from Perfluorinated Polyelectrolytes. Angew. Chem. Int. Ed. 2005, 44, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Renneckar, S.; Hindman, D.P. Nanocomposite-Based Lignocellulosic Fibers 1. Thermal Stability of Modified Fibers with Clay-Polyelectrolyte Multilayers. Cellulose 2007, 15, 333. [Google Scholar] [CrossRef]
- Lahmani, M.; Philippe, H. Nanoscience: Nanobiotechnology and Nanobiology; European Materials Research Society: Strasbourg, France, 2010. [Google Scholar]
- Song, H.; Sun, B.; Gu, K.-J.; Yang, Y.; Zhang, Y.; Shen, Q.-D. Interactions Between Cationic Conjugated Polyelectrolyte and DNA and a Label-Free Method for DNA Detection Based on Conjugated Polyelectrolyte Complexes. J. Appl. Polym. Sci. 2009, 114, 1278–1286. [Google Scholar] [CrossRef]
- Saurer, E.M.; Flessner, R.M.; Sullivan, S.P.; Prausnitz, M.R.; Lynn, D.M. Layer-by-layer assembly of DNA- and protein-containing films on microneedles for drug delivery to the skin. Biomacromolecules 2010, 11(11), 3136–3143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aytar, B.S.; Prausnitz, M.R.; Lynn, D.M. Rapid Release of Plasmid DNA from Surfaces Coated with Polyelectrolyte Multilayers Promoted by the Application of Electrochemical Potentials. ACS Appl. Mater. Interfaces 2012, 4, 2726–2734. [Google Scholar] [CrossRef]
- Iost, R.M.; Crespilho, F.N. Layer-by-Layer Self-Assembly and Electrochemistry: Applications in Biosensing And Bioelectronics. Biosens. Bioelectron. 2012, 31, 1–10. [Google Scholar] [CrossRef]
- Krywko-Cendrowska, A.; Marot, L.; Mathys, D.; Boulmedais, F. Electrochemically-Assisted Self-Assembly of Ion-Imprinted Supramolecular Nanofilms Based on Tannic Acid and Silver Nanoparticles Tailor-Designed for Sensing of Al(III). to be submitted. ACS Appl. Mater. Interfaces.
- Krywko-Cendrowska, A. Voltammetric Tracing of Aluminum Using Supramolecular Metal-Polyphenolic Nanofilms Obtained via Electrochemically Assisted Self-Assembly. CHIMIA 2020, in press. [Google Scholar]
- Blyakhman, F.; Safronov, A.; Shklyar, T. Biomimetic Sensors of the Mechanoelectrical Transduction Based on the Polyelectrolyte Gels. Key Eng. Mater. 2015, 644, 4–7. [Google Scholar] [CrossRef]
- Abdelkebir, K.; Gaudiere, F.; Morin-Grognet, S.; Coquerel, G.; Atmani, H.; Labat, B.; Ladam, G. Protein-Triggered Instant Disassembly of Biomimetic Layer-by-Layer Films. Langmuir 2011, 27, 14370–14379. [Google Scholar] [CrossRef] [PubMed]
- Toutianoush, A.; Krasemann, L.; Tieke, B. Polyelectrolyte Multilayer Membranes for Pervaporation Separation of Alcohol/Water Mixtures. Colloids Surf. A Physicochem. Eng. Asp. 2002, 198, 881–889. [Google Scholar] [CrossRef]
- Chen, Y.; Xiangli, F.; Jin, W.; Xu, N. Organic–Inorganic Composite Pervaporation Membranes Prepared by Self-Assembly of Polyelectrolyte Multilayers on Macroporous Ceramic Supports. J. Membr. Sci. 2007, 2, 78–86. [Google Scholar] [CrossRef]
- Hong, S.U.; Malaisamy, R.; Bruening, M.L. Optimization of Flux and Selectivity in Cl−/SO42− Separations with Multilayer Polyelectrolyte Membranes. J. Membr. Sci. 2006, 2, 366–372. [Google Scholar] [CrossRef]
- Jin, W.; Toutianoush, A.; Tieke, B. Use of Polyelectrolyte Layer-by-Layer Assemblies as Nanofiltration and Reverse Osmosis Membranes. Langmuir 2003, 19, 2550–2553. [Google Scholar] [CrossRef]
- Zhao, F.-Y.; An, Q.-F.; Ji, Y.-L.; Gao, C.-J. A Novel Type of Polyelectrolyte Complex/Mwcnt Hybrid Nanofiltration Membranes for Water Softening. J. Membr. Sci. 2015, 492, 412–421. [Google Scholar] [CrossRef]
- Liu, X.; Bruening, M.L. Size-Selective Transport of Uncharged Solutes through Multilayer Polyelectrolyte Membranes. Chem. Mater. 2004, 16, 351–357. [Google Scholar] [CrossRef]
- Patrício, S.; Cruz, A.I.; Biernacki, K.; Ventura, J.; Eaton, P.; Magalhães, A.L.; Moura, C.; Hillman, A.R.; Freire, C. Novel Layer-by-Layer Interfacial [Ni(Salen)]−Polyelectrolyte Hybrid Films. Langmuir 2010, 26, 10842–10853. [Google Scholar] [CrossRef]
- Frau, A.F.; Thomas, J.; Lane, A.E.S.; Park, J.Y.; Advincula, R.C. Modulating Electrochemical Activity in Polyaniline/Titanium Oxide Hybrid Nanostructured Ultrathin Films. Ind. Eng. Chem. Res. 2011, 50, 5532–5542. [Google Scholar] [CrossRef]
- Priya, D.; Neela, J.M.M.; Trebše, P.; Žabar, R.; Raichur, A.M. Photocatalytic Degradation of Dimethoate Using Lbl Fabricated Tio2/Polymer Hybrid Films. J. Hazard. Mater. 2011, 195, 214–222. [Google Scholar] [CrossRef]
- Chen, D.; Wang, G.; Lu, W.; Zhang, H.; Li, J. Photoelectrochemical Study of Organic–Inorganic Hybrid Thin Films via Electrostatic Layer-by-Layer Assembly. Electrochem. Commun. 2007, 9, 2151–2156. [Google Scholar] [CrossRef]
- Zhang, Q.; Atay, T.; Tischler, J.R.; Bradley, M.S.; Bulović, V.; Nurmikko, A.V. Highly Efficient Resonant Coupling of Optical Excitations in Hybrid Organic/Inorganic Semiconductor Nanostructures. Nat. Nanotechnol. 2007, 2, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Sewell, S.L.; Wright, D.W. Biomimetic Synthesis of Titanium Dioxide Utilizing the R5 Peptide Derived from Cylindrotheca fusiformis. Chem. Mater. 2006, 18(13), 3108–3113. [Google Scholar] [CrossRef]
- Sakai, N.; Prasad, G.K.; Ebina, Y.; Takada, K.; Sasaki, T. Layer-by-Layer Assembled TiO2 Nanoparticle/Pedot-Pss Composite Films for Switching of Electric Conductivity in Response to Ultraviolet and Visible Light. Chem. Mater. 2006, 18, 3596–3598. [Google Scholar] [CrossRef]
- Johnston, A.P.R.; Read, E.S.; Caruso, F. DNA Multilayer Films on Planar and Colloidal Supports: Sequential Assembly of Like-Charged Polyelectrolytes. Nano Lett. 2005, 5, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Mascaro, L.H.; Gonçalves, D.; Bulhões, L.O.S. Electrocatalytic Properties and Electrochemical Stability of Polyaniline and Polyaniline Modified with Platinum Nanoparticles in Formaldehyde Medium. Thin Solid Film. 2004, 461, 243–249. [Google Scholar] [CrossRef]
- O’Mullane, A.P.; Dale, S.E.; Day, T.M.; Wilson, N.R.; Macpherson, J.V.; Unwin, P.R. Formation of Polyaniline/Pt Nanoparticle Composite Films and Their Electrocatalytic Properties. J. Solid State Electrochem. 2006, 10, 792–807. [Google Scholar] [CrossRef]
- Hepel, M. The Electrocatalytic Oxidation of Methanol at Finely Dispersed Platinum Nanoparticles in Polypyrrole Films. J. Electrochem. Soc. 1998, 145, 124. [Google Scholar] [CrossRef]
- Tian, S.; Liu, J.; Zhu, T.; Knoll, W. Polyaniline/Gold Nanoparticle Multilayer Films: Assembly, Properties.; Biological Applications. Chem. Mater. 2004, 16, 4103–4108. [Google Scholar] [CrossRef]
- Kulesza, P.J.; Chojak, M.; Karnicka, K.; Mieznikowski, K.; Palys, B.; Lewera, A.; Wieckowski, A. Network Films Composed of Conducting Polymer-Linked and Polyoxometalate-Stabilized Platinum Nanoparticles. Chem. Mater. 2004, 16, 4128–4134. [Google Scholar] [CrossRef]
- Sheeney-Haj-Ichia, L.; Sharabi, G.; Willner, I. Control of the Electronic Properties of Thermosensitive Poly (N-isopropylacrylamide) and Au-Nano-particle/Poly (N-isopropylacrylamide) Composite Hydrogels upon Phase Transition. Adv. Funct. Mater. 2002, 12, 27–32. [Google Scholar] [CrossRef]
- Gaponik, N.P.; Talapin, D.V.; Rogach, A.L.; Eychmüller, A. Electrochemical Synthesis of CdTe Nanocrystal/Polypyrrole Composites for Optoelectronic Applications. J. Mater. Chem. 2000, 10, 2163–2166. [Google Scholar] [CrossRef]
- Khanna, P.K.; Lonkar, S.P.; Subbarao, V.V.V.S.; Jun, K.-W. Polyaniline–CdS Nanocomposite from Organometallic Cadmium Precursor. Mater. Chem. Phys. 2004, 87, 49–52. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, M. Polyaniline/TiO2 Composite Nanotubes. J. Phys. Chem. B 2003, 107, 6748–6753. [Google Scholar] [CrossRef]
- Granot, E.; Patolsky, F.; Willner, I. Electrochemical Assembly of a CdS Semiconductor Nanoparticle Monolayer on Surfaces: Structural Properties and Photoelectrochemical Applications. J. Phys. Chem. B 2004, 108, 5875–5881. [Google Scholar] [CrossRef]
- Pardo-Yissar, V.; Bourenko, T.; Wasserman, J.; Willner, I. Solvent-Switchable Photoelectrochemistry in the Presence of CdS-Nanoparticle/Acrylamide Hydrogels. Adv. Mater. 2002, 14, 670–673. [Google Scholar] [CrossRef]
- García, M.C. Drug Delivery Systems Based on Nonimmunogenic Biopolymers. In Engineering of Biomaterials for Drug Delivery Systems; Woodhead Publishing: Sawston, UK, 2018; pp. 317–344. [Google Scholar]
- Van der Maarel, J.R.C. Introduction to Biopolymer Physics; World Scientific Publishing Company: Cambridge, UK; Singapore, 2007. [Google Scholar]
- Gu, Y.; Huang, J. Ultrathin Cellulose Film Coating of Porous Alumina Membranes for Adsorption of Superoxide Dismutase. J. Mater. Chem. B 2013, 1, 5636–5643. [Google Scholar] [CrossRef]
- Garza, J.M.; Jessel, N.; Ladam, G.; Dupray, V.; Muller, S.; Stoltz, J.; Schaaf, P.; Voegel, J.; Lavalle, P. Polyelectrolyte Multilayers and Degradable Polymer Layers as Multicompartment Films. Langmuir 2005, 21, 12372–12377. [Google Scholar] [CrossRef]
- Tezcaner, A.; Hicks, D.; Boulmedais, F.; Sahel, J.; Schaaf, P.; Voegel, J.; Lavalle, P. Polyelectrolyte Multilayer Films as Substrates for Photoreceptor Cells. Biomacromolecules 2006, 7, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Chirea, M.; Vladimir, G.-M.; Manzanares, J.A.; Pereira, C.; Gulaboski, R.; Silva, F. Electrochemical Characterization of Polyelectrolyte/Gold Nanoparticle Multilayers Self-Assembled on Gold Electrodes. J. Phys. Chem. B 2005, 109, 21808–21817. [Google Scholar] [CrossRef] [Green Version]
- Granot, E.; Katz, E.; Basnar, B.; Willner, I. Enhanced Bioelectrocatalysis Using Au-Nanoparticle/Polyaniline Hybrid Systems in Thin Films and Microstructured Rods Assembled on Electrodes. Chem. Mater. 2005, 17, 4600–4609. [Google Scholar] [CrossRef]
- Dowdy, C.E.; Leopold, M.C. Enhanced Electrochemistry of Nanoparticle-Embedded Polyelectrolyte Films: Interfacial Electronic Coupling and Distance Dependence. Thin Solid Film. 2010, 519, 790–796. [Google Scholar] [CrossRef]
- Lyubartsev, A.P.; Nordenskiöld, L. Monte Carlo Simulation Study of DNA Polyelectrolyte Properties in the Presence of Multivalent Polyamine Ions. J. Phys. Chem. B 1997, 101, 4335–4342. [Google Scholar] [CrossRef]
- Seručnik, M.; Podlipnik, C.; Barbara, H.-L. DNA–Polyelectrolyte Complexation Study: The Effect of Polyion Charge Density and Chemical Nature of the Counterions. J. Phys. Chem. B 2018, 122, 5381–5388. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.S.; Pun, S.H. Extracellular Barriers to in vivo PEI and PEGylated PEI Polyplex-Mediated Gene Delivery to the Liver. Bioconj. Chem. 2008, 19, 693–704. [Google Scholar] [CrossRef] [PubMed]
- DeMuth, P.C.; Min, Y.; Huang, B.; Kramer, J.A.; Miller, A.D.; Barouch, D.H.; Hammond, P.T.; Irvine, D.J. Polymer Multilayer Tattooing for Enhanced DNA Vaccination. Nat. Mater. 2013, 12, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Sterner, E.S.; Coughlin, E.B.; Theato, P. o-Nitrobenzyl Alcohol Derivatives: Opportunities in Polymer and Materials Science. Macromolecules 2012, 45, 1723–1736. [Google Scholar] [CrossRef]
- Il’ichev, Y.V.; Schwörer, M.A.; Wirz, J. Photochemical Reaction Mechanisms of 2-Nitrobenzyl Compounds: Methyl Ethers and Caged ATP. J. Am. Chem. Soc. 2004, 126, 4581–4595. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-Based Hydrogels for Controlled, Localized Drug Delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Schatz, C.; Lucas, J.-M.; Viton, C.; Domard, A.; Pichot, C.; Delair, T. Formation and Properties of Positively Charged Colloids Based on Polyelectrolyte Complexes of Biopolymers. Langmuir 2004, 20, 7766–7778. [Google Scholar] [CrossRef]
- Liu, B.; Bazan, G.C. Conjugated Polyelectrolytes: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Spoto, G.; Corradini, R. Detection of Non-Amplified Genomic DNA; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- He, F.; Feng, F.; Duan, X.; Wang, S.; Li, Y.; Zhu, D. Selective and Homogeneous Fluorescent DNA Detection by Target-Induced Strand Displacement Using Cationic Conjugated Polyelectrolytes. Anal. Chem. 2008, 80(6), 2239–2243. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Fujita, M.Y.; Takeoka, Y.; Rikukawa, M. Formation of Polyelectrolyte Complexes from Cationic Polyfluorenes and Ssdna. J. Anal. Bioanal. Tech. 2017, 8, 1000388. [Google Scholar] [CrossRef]
- Xia, F.; Zuo, X.; Yang, R.; Xiao, Y.; Kang, D.; Alexis, V.-B.; Gong, X.; Yuen, J.D.; Hsu, B.B.; Heeger, A.J.; et al. Colorimetric Detection of DNA, Small Molecules, Proteins.; Ions Using Unmodified Gold Nanoparticles and Conjugated Polyelectrolytes. Proc. Natl. Acad. Sci. USA 2010, 107, 10837–10841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhan, R.; Li, T.; Pu, K.; Wang, Y.; Tan, Y.C.; Liu, B. Fluorescence and Visual Detection of Single Nucleotide Polymorphism Using Cationic Conjugated Polyelectrolyte. Langmuir 2012, 28, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Rozhanchuk, T.; Mariia, V.; Titov, M.; Tananaiko, O. Voltammetric Determination of Purine Bases Using a Carbon Electrode Modified with Hybrid Silica Film. Electroanalysis 2013, 25, 2045–2053. [Google Scholar] [CrossRef]
- Sun, B.; Mutch, S.A.; Lorenz, R.M.; Chiu, D.T. Layered Polyelectrolyte−Silica Coating for Nanocapsules. Langmuir 2005, 21, 10763–10769. [Google Scholar] [CrossRef]
- Wu, S.-H.; Hung, Y.; Mou, C.-Y. Mesoporous Silica Nanoparticles as Nanocarriers. Chem. Commun. 2011, 47, 9972–9985. [Google Scholar] [CrossRef]
- Davies, G.-L.; Antoinette, B.; Yurii, K.; Gun, K.O. Preparation and Size Optimisation of Silica Nanoparticles Using Statistical Analyses. Chem. Phys. Lett. 2009, 468, 239–244. [Google Scholar] [CrossRef]
- Erni, P.; Dardelle, G.; Sillick, M.; Wong, K.; Beaussoubre, P.; Fieber, W. Turning Coacervates into Biohybrid Glass: Core/Shell Capsules Formed by Silica Precipitation in Protein/Polysaccharide Scaffolds. Angew. Chem. Int. Ed. 2013, 52, 10334–10338. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, Z.; Wang, C.; Tong, Z. Versatile Fabrication of Nanocomposite Microcapsules with Controlled Shell Thickness and Low Permeability. ACS Appl. Mater. Interfaces 2013, 5, 2495–2502. [Google Scholar] [CrossRef]
- Niu, Z.; Yang, Z.; Hu, Z.; Lu, Y.; Han, C.C. Polyaniline–Silica Composite Conductive Capsules and Hollow Spheres. Adv. Funct. Mater. 2003, 13, 949–954. [Google Scholar] [CrossRef]
- Elfwing, A.; Cai, W.; Ouyang, L.; Liu, X.; Xia, Y.; Tang, Z.; Inganäs, O. DNA Based Hybrid Material for Interface Engineering in Polymer Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 9579–9586. [Google Scholar] [CrossRef] [PubMed]
- Ladam, G.; Schaaf, P.; Cuisinier, F.J.G.; Decher, G.; Voegel, J. Protein Adsorption onto Auto-Assembled Polyelectrolyte Films. Langmuir 2001, 17, 878–882. [Google Scholar] [CrossRef]
- Schwinté, P.; Voegel, J.C.; Picart, C.; Haikel, Y.; Schaaf, P.; Szalontai, B. Stabilizing Effects of Various Polyelectrolyte Multilayer Films on the Structure of Adsorbed/Embedded Fibrinogen Molecules: An Atr−Ftir Study. J. Phys. Chem. B 2001, 105, 11906–11916. [Google Scholar] [CrossRef]
- Jessel, N.; Atalar, F.; Lavalle, P.; Mutterer, J.; Decher, G.; Schaaf, P.; Voegel, J.-C.; Ogier, J. Bioactive Coatings Based on a Polyelectrolyte Multilayer Architecture Functionalized by Embedded Proteins. Adv. Mater. 2003, 15, 692–695. [Google Scholar] [CrossRef]
- Szyk, L.; Schaaf, P.; Gergely, C.; Voegel, J.C.; Tinland, B. Lateral Mobility of Proteins Adsorbed on or Embedded in Polyelectrolyte Multilayers. Langmuir 2001, 17, 6248–6253. [Google Scholar] [CrossRef]
- Szyk, L.; Schwinte, P.; Voegel, J.C.; Schaaf, P.; Tinland, B. Dynamical Behavior of Human Serum Albumin Adsorbed on or Embedded in Polyelectrolyte Multilayers. J. Phys. Chem. B 2002, 106, 6049–6055. [Google Scholar] [CrossRef]
- Zürcher, S.; Wäckerlin, D.; Bethuel, Y.; Malisova, B.; Textor, M.; Tosatti, S.; Gademann, K. Biomimetic Surface Modifications Based on the Cyanobacterial Iron Chelator Anachelin. J. Am. Chem. Soc. 2006, 128, 1064–1065. [Google Scholar] [CrossRef]
- Öner, M.; Doğan, Ö. Inhibitory Effect of Polyelectrolytes on Crystallization Kinetics of Hydroxyapatite. Prog. Cryst. Growth Charact. Mater. 2005, 3, 39–51. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, J. Protein Microarrays on Hybrid Polymeric Thin Films Prepared by Self-Assembly of Polyelectrolytes for Multiple-Protein Immunoassays. Proteomics 2006, 6, 1415–1426. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, J. Antibody-Microarrays on Hybrid Polymeric Thin Film-Coated Slides for Multiple-Protein Immunoassays. In Microarrays: Volume 2: Applications and Data Analysis; Rampal, J.B., Ed.; Humana Press: Totowa, NJ, YSA, 2007; pp. 259–271. [Google Scholar]
- Serpe, M.J.; Yarmey, K.A.; Nolan, C.M.; Lyon, L.A. Doxorubicin Uptake and Release from Microgel Thin Films. Biomacromolecules 2005, 6, 408–413. [Google Scholar] [CrossRef]
- Hajicharalambous, C.S.; Lichter, J.; Hix, W.T.; Swierczewska, M.; Rubner, M.F.; Rajagopalan, P. Nano-and Sub-Micron Porous Polyelectrolyte Multilayer Assemblies: Biomimetic Surfaces for Human Corneal Epithelial Cells. Biomaterials 2009, 24, 4029–4036. [Google Scholar] [CrossRef] [PubMed]
- Svaldo Lanero, T.; Cavalleri, O.; Krol, S.; Rolandi, R.; Gliozzi, A. Mechanical Properties of Single Living Cells Encapsulated in Polyelectrolyte Matrixes. J. Biotechnol. 2006, 124, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.-H.; Wang, H.-C.; Kuan, C.-H.; Chen, M.-H.; Wu, H.-C.; Sun, J.-S.; Wang, T.-W. Glycosaminoglycan-Based Hybrid Hydrogel Encapsulated with Polyelectrolyte Complex Nanoparticles for Endogenous Stem Cell Regulation in Central Nervous System Regeneration. Biomaterials 2018, 174, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Giussi, J.M.; Cortez, M.L.; Marmisollé, W.A.; Azzaroni, O. Practical Use of Polymer Brushes in Sustainable Energy Applications: Interfacial Nanoarchitectonics for High-Efficiency Devices. Chem. Soc. Rev. 2019, 48, 814–849. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Imbrogno, J.; Belfort, G.; Wang, X.-L. Making Polymeric Membranes Antifouling via “Grafting from” Polymerization of Zwitterions. J. Appl. Polym. Sci. 2015, 132, 41781. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Miller, P.J.; Shukla, N.; Immaraporn, B.; Gelman, A.; Luokala, B.B.; Siclovan, T.M.; Kickelbick, G.; Vallant, T.; Hoffmann, H. Polymers at Interfaces: Using Atom Transfer Radical Polymerization in the Controlled Growth of Homopolymers and Block Copolymers from Silicon Surfaces in the Absence of Untethered Sacrificial Initiator. Macromolecules 1999, 32, 8716–8724. [Google Scholar] [CrossRef]
- Baum, M.; Brittain, W.J. Synthesis of Polymer Brushes on Silicate Substrates via Reversible Addition Fragmentation Chain Transfer Technique. Macromolecules 2002, 35, 610–615. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New Polymer Synthesis by Nitroxide Mediated Living Radical Polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef]
- De Boer, B.; Simon, H.K.; Werts, M.P.L.; van der Vegte, E.W.; Hadziioannou, G. “Living” Free Radical Photopolymerization Initiated from Surface-Grafted Iniferter Monolayers. Macromolecules 2000, 33, 349–356. [Google Scholar] [CrossRef]
- Chen, W.-L.; Cordero, R.; Tran, H.; Ober, C.K. 50th Anniversary Perspective: Polymer Brushes: Novel Surfaces for Future Materials. Macromolecules 2017, 50, 4089–4113. [Google Scholar] [CrossRef]
- Krishnamoorthy, M.; Hakobyan, S.; Ramstedt, M.; Gautrot, J.E. Surface-Initiated Polymer Brushes in the Biomedical Field: Applications in Membrane Science, Biosensing; Cell Culture, Regenerative Medicine and Antibacterial Coatings. Chem. Rev. 2014, 114, 10976–11026. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, S.; Osborne, V.L.; Huck, W.T.S. Polymer Brushes via Surface-Initiated Polymerizations. Chem. Soc. Rev. 2004, 33, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Barbey, R.; Lavanant, L.; Paripovic, D.; Schuwer, N.; Sugnaux, C.; Tugulu, S.; Klok, H.-A. Polymer Brushes via Surface-Initiated Controlled Radical Polymerization: Synthesis, Characterization; Properties Applications. Chem. Rev. 2009, 109, 5437–5527. [Google Scholar] [CrossRef] [PubMed]
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.-A. Surface-Initiated Controlled Radical Polymerization: State-Of-The-Art, Opportunities.; Challenges in Surface and Interface Engineering with Polymer Brushes. Chem. Rev. 2017, 117, 1105–1318. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zan, F.; Ke, Y.; Wu, G. Cells May Feel a Hard Substrate Even on a Grafted Layer of Soft Hydrogel. J. Mater. Chem. B 2018, 6, 1734–1743. [Google Scholar] [CrossRef]
- Zhao, C.; Li, L.; Wang, Q.; Yu, Q.; Zheng, J. Effect of Film Thickness on The Antifouling Performance of Poly (Hydroxy-Functional Methacrylates) Grafted Surfaces. Langmuir 2011, 27, 4906–4913. [Google Scholar] [CrossRef]
- Huang, T.; Xiao, J.; Wang, S.; Liao, Z.; Huang, T.; Gu, R.; Li, J.; Wu, G.; Liao, H. The Thickness of Poly-Phenoxyethyl Methacrylate Brush Interferes with Cellular Behavior and Function of Myofibers. J. Biomed. Mater. Res. Part. A 2019, 107, 1264–1272. [Google Scholar] [CrossRef]
- Fang, Y.; Xu, W.; Meng, X.-L.; Ye, X.-Y.; Wu, J.; Xu, Z.-K. Poly (2-Hydroxyethyl Methacrylate) Brush Surface for Specific and Oriented Adsorption of Glycosidases. Langmuir 2012, 28, 13318–13324. [Google Scholar] [CrossRef]
- Ejaz, M.; Yamamoto, S.; Ohno, K.; Tsujii, Y.; Fukuda, T. Controlled Graft Polymerization of Methyl Methacrylate on Silicon Substrate by The Combined Use of the Langmuir—Blodgett and Atom Transfer Radical Polymerization Techniques. Macromolecules 1998, 31, 5934–5936. [Google Scholar] [CrossRef]
- Sedjo, R.A.; Mirous, B.K.; Brittain, W.J. Synthesis of Polystyrene-Block-Poly (Methyl Methacrylate) Brushes by Reverse Atom Transfer Radical Polymerization. Macromolecules 2000, 33, 1492–1493. [Google Scholar] [CrossRef]
- Barbey, R.; Kauffmann, E.; Ehrat, M.; Klok, H. Protein Microarrays Based on Polymer Brushes Prepared Via Surface-Initiated Atom Transfer Radical Polymerization. Biomacromolecules 2010, 11, 3467–3479. [Google Scholar] [CrossRef] [PubMed]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living Free-Radical Polymerization by Reversible Addition−Fragmentation Chain Transfer: The RAFT Process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Barner, L.; Christopher, B.-K.; Davis, T.P.; Stenzel, M.H. Complex Molecular Architecture Polymers via RAFT. Aust. J. Chem. 2004, 57, 19–24. [Google Scholar] [CrossRef]
- Demirci, S.; Caykara, T. Controlled Grafting of Cationic Poly [(Ar-Vinylbenzyl) Trimethylammonium Chloride] on Hydrogen-Terminated Silicon Substrate by Surface-Initiated RAFT Polymerization. React. Funct. Polym. 2012, 72, 588–595. [Google Scholar] [CrossRef]
- Kitano, H.; Liu, Y.; Tokuwa, K.; Li, L.; Iwanaga, S.; Nakamura, M.; Kanayama, N.; Ohno, K.; Saruwatari, Y. Polymer Brush with Pendent Glucosylurea Groups Constructed on a Glass Substrate by RAFT Polymerization. Eur. Polym. J. 2012, 48, 1875–1882. [Google Scholar] [CrossRef]
- Kondo, T.; Gemmei-Ide, M.; Kitano, H.; Ohno, K.; Noguchi, H.; Uosaki, K. Sum frequency Generation Study on the Structure of Water in the Vicinity of an Amphoteric Polymer Brush. Colloids Surf. B Biointerfaces 2012, 91, 215–218. [Google Scholar] [CrossRef]
- Moad, G.; Chong, Y.K.; Postma, A.; Rizzardo, E.; Thang, S.H. Advances in RAFT Polymerization: The Synthesis of Polymers with Defined End-Groups. Polymer 2005, 46, 8458–8468. [Google Scholar] [CrossRef]
- Scales, C.W.; Vasilieva, Y.A.; Convertine, A.J.; Lowe, A.B.; McCormick, C.L. Direct, Controlled Synthesis of The Nonimmunogenic, Hydrophilic Polymer, Poly (N-(2-Hydroxypropyl) Methacrylamide) via RAFT in Aqueous Media. Biomacromolecules 2005, 6, 1846–1850. [Google Scholar] [CrossRef]
- Rowe-Konopacki, M.D.; Boyes, S.G. Synthesis of Surface Initiated Diblock Copolymer Brushes from Flat Silicon Substrates Utilizing the RAFT Polymerization Technique. Macromolecules 2007, 40, 879–888. [Google Scholar] [CrossRef]
- Zhai, G.; Yu, W.H.; Kang, E.T.; Neoh, K.G.; Huang, C.C.; Liaw, D.J. Functionalization of Hydrogen-Terminated Silicon with Polybetaine Brushes via Surface-Initiated Reversible Addition− Fragmentation Chain-Transfer (RAFT) Polymerization. Ind. Eng. Chem. Res. 2004, 43, 1673–1680. [Google Scholar] [CrossRef]
- Yuan, J.; Huang, X.; Li, P.; Li, L.; Shen, J. Surface-Initiated RAFT Polymerization of Sulfobetaine from Cellulose Membranes to Improve Hemocompatibility and Antibiofouling Property. Polym. Chem. 2013, 4, 5074–5085. [Google Scholar] [CrossRef]
- Hu, Q.; Kong, J.; Han, D.; Zhang, Y.; Bao, Y.; Zhang, X.; Niu, L. Electrochemically Controlled RAFT Polymerization for Highly Sensitive Electrochemical Biosensing of Protein Kinase Activity. Anal. Chem. 2019, 91, 1936–1943. [Google Scholar] [CrossRef] [PubMed]
- Hawker, C.J. “Living” Free Radical Polymerization: A Unique Technique for the Preparation of Controlled Macromolecular Architectures. Acc. Chem. Res. 1997, 30, 373–382. [Google Scholar] [CrossRef]
- Sciannamea, V.; Jérôme, R.; Detrembleur, C. In-Situ Nitroxide-Mediated Radical Polymerization (NMP) Processes: Their Understanding and Optimization. Chem. Rev. 2008, 3, 1104–1126. [Google Scholar] [CrossRef]
- Husseman, M.; Malmström, E.E.; McNamara, M.; Mate, M.; Mecerreyes, D.; Benoit, D.G.; Hedrick, J.L.; Mansky, P.; Huang, E.; Russell, T.P.; et al. Controlled Synthesis of Polymer Brushes by “Living” Free Radical Polymerization Techniques. Macromolecules 1999, 32, 1424–1431. [Google Scholar] [CrossRef]
- Parvole, J.; Montfort, J.-P.; Reiter, G.; Borisov, O.; Billon, L. Elastomer Polymer Brushes on Flat Surface by Bimolecular Surface-Initiated Nitroxide Mediated Polymerization. Polymer 2006, 47, 972–981. [Google Scholar] [CrossRef]
- Husemann, M.; Morrison, M.; Benoit, D.; Frommer, J.; Mate, C.M.; Hinsberg, W.D.; Hedrick, J.L.; Hawker, C.J. Manipulation of Surface Properties by Patterning of Covalently Bound Polymer Brushes. J. Am. Chem. Soc. 2000, 122, 1844–1845. [Google Scholar] [CrossRef]
- Grubbs, R.B. Nitroxide-Mediated Radical Polymerization: Limitations and Versatility. Polym. Rev. 2011, 2, 104–137. [Google Scholar] [CrossRef]
- Otsu, T. Iniferter Concept and Living Radical Polymerization. J. Polym. Sci. Part. A Polym. Chem. 2000, 38, 2121–2136. [Google Scholar] [CrossRef]
- Yan, J.; Li, B.; Zhou, F.; Liu, W. Ultraviolet Light-Induced Surface-Initiated Atom-Transfer Radical Polymerization. ACS Macro Lett. 2013, 2, 592–596. [Google Scholar] [CrossRef]
- Li, A.; Benetti, E.M.; Tranchida, D.; Clasohm, J.N.; Schönherr, H.; Spencer, N.D. Surface-Grafted, Covalently Cross-Linked Hydrogel Brushes with Tunable Interfacial and Bulk Properties. Macromolecules 2011, 44, 5344–5351. [Google Scholar] [CrossRef]
- Dulay, M.T.; Baca, Q.J.; Zare, R.N. Enhanced Proteolytic Activity of Covalently Bound Enzymes in Photopolymerized Sol Gel. Anal. Chem. 2005, 77, 4604–4610. [Google Scholar] [CrossRef]
- Kaya, N.U.; Onen, A.; Guvenilir, Y. Photopolymerization of Acrylates by Enzymatically Synthesized PCL Based Macrophotoinitiator. Express Polym. Lett. 2017, 11. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Yagci, Y. Light-Induced Click Reactions. Angew. Chem. Int. Ed. 2013, 52, 5930–5938. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, G.; Stansbury, J.W.; Zhu, X.; Wang, X.; Nie, J. Smart Antibacterial Surface Made by Photopolymerization. ACS Appl. Mater. Interfaces 2016, 8, 28047–28054. [Google Scholar] [CrossRef] [PubMed]
- Bayramoğlu, G.; Arıca, M.Y. Immobilization of Laccase onto Poly (Glycidylmethacrylate) Brush Grafted Poly (Hydroxyethylmethacrylate) Films: Enzymatic Oxidation of Phenolic Compounds. Mater. Sci. Eng. C 2009, 29, 1990–1997. [Google Scholar] [CrossRef]
- Zdyrko, B.; Luzinov, I. Polymer Brushes by the “Grafting To” method. Macromol. Rapid Commun. 2011, 32, 859–869. [Google Scholar] [CrossRef]
- Lee, H.-S.; Penn, L.S. In Situ Study of Polymer Brushes as Selective Barriers to Diffusion. Macromolecules 2008, 41, 8124–8129. [Google Scholar] [CrossRef]
- Belegrinou, S.; Jan, D.; Kreiter, M.; Katarzyna, K.-T.; Sinner, E.-K.; Meier, W. Biomimetic Supported Membranes from Amphiphilic Block Copolymers. Soft Matter 2010, 6, 179–186. [Google Scholar] [CrossRef]
- Motornov, M.; Sheparovych, R.; Lupitskyy, R.; MacWilliams, E.; Hoy, O.; Luzinov, I.; Minko, S. Stimuli-Responsive Colloidal Systems from Mixed Brush-Coated Nanoparticles. Adv. Funct. Mater. 2007, 17, 2307–2314. [Google Scholar] [CrossRef]
- Zhao, W.; Krausch, G.; Rafailovich, M.H.; Sokolov, J. Lateral Structure of a Grafted Polymer Layer in a Poor Solvent. Macromolecules 1994, 27, 2933–2935. [Google Scholar] [CrossRef]
- Jones, R.A.L.; Lehnert, R.J.; Schonherr, H.; Vancso, J. Factors Affecting the Preparation of Permanently End-Grafted Polystyrene Layers. Polymer 1999, 40, 525–530. [Google Scholar] [CrossRef]
- Corbierre, M.K.; Cameron, N.S.; Sutton, M.; Laaziri, K.; Lennox, R.B. Gold Nanoparticle/Polymer Nanocomposites: Dispersion of Nanoparticles as a Function of Capping Agent Molecular Weight and Grafting Density. Langmuir 2005, 21, 6063–6072. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.; Jones, R.A.L. Producing High-Density High-Molecular-Weight Polymer Brushes by a “Grafting To” Method from a Concentrated Homopolymer Solution. Langmuir 2010, 26, 13954–13958. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Nuopponen, M.; Jiang, H.; Viitala, T.; Kauppinen, E.; Kontturi, K.; Tenhu, H. Amphiphilic Gold Nanoparticles Grafted with Poly (N-Isopropylacrylamide) and Polystyrene. Macromolecules 2005, 38, 2918–2926. [Google Scholar] [CrossRef]
- Liu, G.; Yan, L.; Chen, X.; Zhang, G. Study of the Kinetics of Mushroom-to-Brush Transition of Charged Polymer Chains. Polymer 2006, 47, 3157–3163. [Google Scholar] [CrossRef]
- Vyas, M.K.; Nandan, B.; Schneider, K.; Stamm, M. Nanowear Studies in Chemically Heterogeneous Responsive Polymeric Brushes by Surface Force Microscopy. Eur. Polym. J. 2009, 45, 1367–1376. [Google Scholar] [CrossRef]
- Motornov, M.; Sheparovych, R.; Tokarev, I.; Roiter, Y.; Minko, S. Nonwettable Thin Films from Hybrid Polymer Brushes Can Be Hydrophilic. Langmuir 2007, 23, 13–19. [Google Scholar] [CrossRef]
- Crooks, R.M. Patterning of Hyperbranched Polymer Films. ChemPhysChem 2001, 2, 644–654. [Google Scholar] [CrossRef]
- De Vos, K.; Girones, J.; Popelka, S.; Schacht, E.; Baets, R.; Bienstman, P. SOI Optical Microring Resonator with Poly (Ethylene Glycol) Polymer Brush for Label-Free Biosensor Applications. Biosens. Bioelectron. 2009, 24, 2528–2533. [Google Scholar] [CrossRef]
- Luzinov, I.; Julthongpiput, D.; Tsukruk, V.V. Stability of Microdomain Morphology in Tethered Block Copolymer Monolayers. Polymer 2001, 42, 2267–2273. [Google Scholar] [CrossRef]
- He, J.; Chen, J.; Hu, G.; Wang, L.; Zheng, J.; Zhan, J.; Zhu, Y.; Zhong, C.; Shi, X.; Liu, S.; et al. Immobilization of an Antimicrobial Peptide on Silicon Surface with Stable Activity by Click Chemistry. J. Mater. Chem. B 2018, 6, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Michalek, L.; Mundsinger, K.; Christopher, B.-K.; Barner, L. The Long and the Short of Polymer Grafting. Polym. Chem. 2019, 10, 54–59. [Google Scholar] [CrossRef]
- Minko, S. Grafting on Solid Surfaces: “Grafting to” and “Grafting from” Methods. In Polymer Surfaces and Interfaces; Springer: Berlin/Heidelberg, Germany, 2008; pp. 215–234. [Google Scholar]
- Pun, C.-C.; Lee, K.; Kim, H.-J.; Kim, J. Signal Amplifying Conjugated Polymer-Based Solid-State DNA Sensors. Macromolecules 2006, 39, 7461–7463. [Google Scholar] [CrossRef]
- Telegdi, J.; Szabó, T.; Románszki, L.; Pávai, M. The Use of Nano-/Microlayers, Self-Healing and Slow-Release Coatings to Prevent Corrosion and Biofouling. In Handbook of Smart Coatings for Materials Protection; Elsevier: Amsterdam, The Netherlands, 2014; pp. 135–182. [Google Scholar] [CrossRef] [Green Version]
- Sato, N.; Ito, S.; Yamamoto, M. Interfacial Behavior of Acetalized Poly (Vinyl Alcohol) Monolayers Observed by Brewster Angle Microscopy and Canal Surface Viscometry. Polym. J. 1996, 28, 784–789. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Huang, Z.; Hua, W.; Castada, H.; Allen, H.C. Reorganization and Caging of DPPC, DPPE, DPPG.; DPPS Monolayers Caused by Dimethylsulfoxide Observed Using Brewster Angle Microscopy. Langmuir 2010, 26, 18902–18908. [Google Scholar] [CrossRef]
- Wales, D.J.; Kitchen, J.A. Surface-Based Molecular Self-Assembly: Langmuir-Blodgett Films of Amphiphilic Ln (III) Complexes. Chem. Cent. J. 2016, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, S.; Ayothi, R.; Hexemer, A.; Finlay, J.A.; Sohn, K.E.; Perry, R.; Ober, C.K.; Kramer, E.J.; Callow, M.E.; Callow, J.A.; et al. Anti-Biofouling Properties of Comblike Block Copolymers with Amphiphilic Side Chains. Langmuir 2006, 22, 5075–5086. [Google Scholar] [CrossRef]
- Draghici, C.; Mikhalevich, V.; Gunkel-Grabole, G.; Kowal, J.; Meier, W.; Palivan, C.G. Biomimetic planar polymer membranes decorated with enzymes as functional surfaces. Langmuir 2018, 34(30), 9015–9024. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Conboy, J.C. Structure of a Gel Phase Lipid Bilayer Prepared by the Langmuir−Blodgett/Langmuir-Schaefer Method Characterized by Sum-Frequency Vibrational Spectroscopy. Langmuir 2005, 21, 9091–9097. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Honma, I.; Zhou, H. Amperometric Biosensor Based on Tyrosinase-Conjugated Polysacchride Hybrid Film: Selective Determination of Nanomolar Neurotransmitters Metabolite of 3, 4-Dihydroxyphenylacetic Acid (DOPAC) in Biological Fluid. Biosens. Bioelectron. 2005, 21, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. Highly Sensitive Electrochemical Immunosensor Based on Polythiophene Polymer with Densely Populated Carboxyl Groups as Immobilization Matrix for Detection of Interleukin 1β In Human Serum and Saliva. Sens. Actuators B Chem. 2018, 270, 18–27. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, L.; Soeller, C.; Travas-Sejdic, J. Conducting Polymers for Electrochemical DNA Sensing. Biomaterials 2009, 30, 2132–2148. [Google Scholar] [CrossRef] [PubMed]
- Adamus, A.; Komasa, J.; Kadłubowski, S.; Ulański, P.; Rosiak, J.M.; Kawecki, M.; Klama-Baryła, A.; Dworak, A.; Trzebicka, B.; Szweda, R. Thermoresponsive Poly [Tri (Ethylene Glycol) Monoethyl Ether Methacrylate]-Peptide Surfaces Obtained by Radiation Grafting-Synthesis and Characterisation. Colloids Surf. B Biointerfaces 2016, 145, 185–193. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Fang, J.; Lu, K.; Wang, Z.; Ma, W.; Wei, Z. Conjugated Polymer–Small Molecule Alloy Leads to High Efficient Ternary Organic Solar Cells. J. Am. Chem. Soc. 2015, 137, 8176–8183. [Google Scholar] [CrossRef]
- Hansen, B.J.; Liu, Y.; Yang, R.; Wang, Z.L. Hybrid Nanogenerator for Concurrently Harvesting Biomechanical and Biochemical Energy. ACS Nano 2010, 4, 3647–3652. [Google Scholar] [CrossRef] [Green Version]
- Gruszkiewicz, A.; Słowikowska, M.; Grześ, G.; Wójcik, A.; Rokita, J.; Fiocco, A.; Wytrwal-Sarna, M.; Marzec, M.; Trzebicka, B.; Kopeć, M.; et al. Enhancement of the Growth of Polymer Brushes Via Atrp Initiated from Ions-Releasing Indium Tin Oxide Substrates. Eur. Polym. J. 2019, 112, 817–821. [Google Scholar] [CrossRef]
- Wolski, K.; Gruszkiewicz, A.; Wytrwal-Sarna, M.; Bernasik, A.; Zapotoczny, S. The Grafting Density and Thickness of Polythiophene-Based Brushes Determine the Orientation, Conjugation Length and Stability of the Grafted Chains. Polym. Chem. 2017, 8, 6250–6262. [Google Scholar] [CrossRef]
- Szuwarzyński, M.; Zaraska, L.; Sulka, G.D.; Zapotoczny, S. Pulsatile Releasing Platform of Nanocontainers Equipped with Thermally Responsive Polymeric Nanovalves. Chem. Mater. 2013, 25, 514–520. [Google Scholar] [CrossRef]
- Jang, J.H.; In, I. Poly (N-Isopropylacrylamide)-Grafted Thermosensitive Anodized Aluminum Oxide Membrane. Chem. Lett. 2010, 39, 1190–1191. [Google Scholar] [CrossRef]
- Fu, Q.; Rao, G.V.R.; Basame, S.B.; Keller, D.J.; Artyushkova, K.; Fulghum, J.E.; López, G.P. Reversible Control of Free Energy and Topography of Nanostructured Surfaces. J. Am. Chem. Soc. 2004, 126, 8904–8905. [Google Scholar] [CrossRef] [PubMed]
- Schlenoff, J.B.; Li, M.; Ly, H. Stability and Self-Exchange in Alkanethiol Monolayers. J. Am. Chem. Soc. 1995, 117, 12528–12536. [Google Scholar] [CrossRef]
- Park, C.S.; Lee, H.J.; Jamison, A.C.; Lee, T.R. Robust Thick Polymer Brushes Grafted from Gold Surfaces Using Bidentate Thiol-Based Atom-Transfer Radical Polymerization Initiators. ACS Appl. Mater. Interfaces 2016, 8, 5586–5594. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, G.; Hu, J.; Lu, X.; Li, J. Temperature, Ionic Strength And Ph Induced Electrochemical Switching Of Smart Polymer Interfaces. Chem. Commun. 2006, 46, 4820–4822. [Google Scholar] [CrossRef]
- Ricci, F.; Lai, R.Y.; Heeger, A.J.; Plaxco, K.W.; Sumner, J.J. Effect of Molecular Crowding on the Response of an Electrochemical DNA Sensor. Langmuir 2007, 23, 6827–6834. [Google Scholar] [CrossRef] [Green Version]
- Radi, A.; Sánchez, J.L.A.; Baldrich, E.; O’Sullivan, C.K. Reagentless, Reusable; Ultrasensitive Electrochemical Molecular Beacon Aptasensor. J. Am. Chem. Soc. 2006, 128, 117–124. [Google Scholar] [CrossRef]
- Peng, S.; Bhushan, B. Smart Polymer Brushes and Their Emerging Applications. RSC Adv. 2012, 23, 8557–8578. [Google Scholar] [CrossRef]
- Carneiro, K.M.M.; Hamblin, G.D.; Hänni, K.D.; Fakhoury, J.; Nayak, M.K.; Rizis, G.; McLaughlin, C.K.; Bazzi, H.S.; Sleiman, H.F. Stimuli-Responsive Organization of Block Copolymers on DNA Nanotubes. Chem. Sci. 2012, 3, 1980–1986. [Google Scholar] [CrossRef]
- Kwak, M.; Gao, J.; Prusty, D.K.; Musser, A.J.; Markov, V.A.; Tombros, N.; Stuart, M.C.A.; Browne, W.R.; Boekema, E.J.; Brinke, G.t.; et al. DNA Block Copolymer Doing It All: From Selection to Self-Assembly of Semiconducting Carbon Nanotubes. Angew. Chem. Int. Ed. 2011, 50, 3206–3210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klonoski, J.; Mondesire, R.; Rea, L.; Ward, D.C.; Jenison, R.D. Enhanced Detection of Staphylococcal Genomes in Positive Blood Cultures Using a Polymeric Enzyme Complex. Anal. Biochem. 2010, 396, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Karaboğa, M.N.S.; Sezgintürk, M.K. Determination of C-reactive Protein by PAMAM Decorated ITO Based Disposable Biosensing System: A New Immunosensor Design from an Old Molecule. Talanta 2018, 186, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ma, Y.; Cui, M.; Luo, X. Enhanced Electrochemical Biosensing of Alpha-Fetoprotein Based on Three-Dimensional Macroporous Conducting Polymer Polyaniline. Sens. Actuators B Chem. 2018, 255, 2568–2574. [Google Scholar] [CrossRef]
- Awsiuk, K.; Stetsyshyn, Y.; Raczkowska, J.; Lishchynskyi, O.; Dąbczyński, P.; Kostruba, A.; Ohar, H.; Shymborska, Y.; Nastyshyn, S.; Budkowski, A. Temperature-Controlled Orientation of Proteins on Temperature-Responsive Grafted Polymer Brushes: Poly (butyl methacrylate) vs Poly (butyl acrylate): Morphology, Wetting.; Protein Adsorption. Biomacromolecules 2019, 20, 2185–2197. [Google Scholar] [CrossRef]
- Likibi, F.; Jiang, B.; Li, B. Biomimetic Nanocoating Promotes Osteoblast Cell Adhesion on Biomedical Implants. J. Mater. Res. 2008, 23, 3222–3228. [Google Scholar] [CrossRef] [Green Version]
- Harris, B.P.; Kutty, J.K.; Fritz, E.W.; Webb, C.K.; Burg, K.J.L.; Metters, A.T. Photopatterned Polymer Brushes Promoting Cell Adhesion Gradients. Langmuir 2006, 22, 4467–4471. [Google Scholar] [CrossRef]
- Moro, T.; Takatori, Y.; Ishihara, K.; Konno, T.; Takigawa, Y.; Matsushita, T.; Chung, U.; Nakamura, K.; Kawaguchi, H. Surface Grafting of Artificial Joints with a Biocompatible Polymer for Preventing Periprosthetic Osteolysis. Nat. Mater. 2004, 3, 829–836. [Google Scholar] [CrossRef]
- Klein, J. Repair or Replacement—A Joint Perspective. Science 2009, 5910, 47–48. [Google Scholar] [CrossRef]
- Kobayashi, M.; Terayama, Y.; Hosaka, N.; Kaido, M.; Suzuki, A.; Yamada, N.; Torikai, N.; Ishihara, K.; Takahara, A. Friction Behavior of High-Density Poly (2-Methacryloyloxyethyl Phosphorylcholine) Brush in Aqueous Media. Soft Matter 2007, 3, 740–746. [Google Scholar] [CrossRef]
- Nomura, A.; Okayasu, K.; Ohno, K.; Fukuda, T.; Tsujii, Y. Lubrication Mechanism of Concentrated Polymer Brushes in Solvents: Effect of Solvent Quality and Thereby Swelling State. Macromolecules 2011, 44, 5013–5019. [Google Scholar] [CrossRef]
- Feng, W.; Gao, X.; McClung, G.; Zhu, S.; Ishihara, K.; Brash, J.L. Methacrylate Polymer Layers Bearing Poly (Ethylene Oxide) and Phosphorylcholine Side Chains as Non-Fouling Surfaces: In Vitro Interactions with Plasma Proteins and Platelets. Acta Biomater. 2011, 7, 3692–3699. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, C.; Goto, A.; Tsujii, Y.; Fukuda, T.; Kimura, T.; Yamamoto, K.; Kishida, A. Protein Repellency of Well-Defined, Concentrated Poly (2-Hydroxyethyl Methacrylate) Brushes by the Size-Exclusion Effect. Macromolecules 2006, 39, 2284–2290. [Google Scholar] [CrossRef]
- Feng, W.; Brash, J.L.; Zhu, S. Non-Biofouling Materials Prepared by Atom Transfer Radical Polymerization Grafting of 2-Methacryloloxyethyl Phosphorylcholine: Separate Effects of Graft Density and Chain Length on Protein Repulsion. Biomaterials 2006, 27, 847–855. [Google Scholar] [CrossRef]
- Zhou, R.; Ren, P.-F.; Yang, H.-C.; Xu, Z.-K. Fabrication of Antifouling Membrane Surface by Poly (Sulfobetaine Methacrylate)/Polydopamine Co-Deposition. J. Membr. Sci. 2014, 466, 18–25. [Google Scholar] [CrossRef]
- Ma, H.; Li, D.; Sheng, X.; Zhao, B.; Chilkoti, A. Protein-Resistant Polymer Coatings on Silicon Oxide by Surface-Initiated Atom Transfer Radical Polymerization. Langmuir 2006, 22, 3751–3756. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, Y.; Wang, B.; Sun, C.; Chakraborty, S.; Ramasubramanian, K.; Dutta, P.K.; Ho, W.S.W. Multilayer Polymer/Zeolite Y Composite Membrane Structure for CO2 Capture from Flue Gas. J. Membr. Sci. 2016, 498, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Hosono, K.; Matsubara, I.; Murayama, N.; Woosuck, S.; Izu, N. Synthesis of Polypyrrole/MoO3 Hybrid Thin Films and Their Volatile Organic Compound Gas-Sensing Properties. Chem. Mater. 2005, 17, 349–354. [Google Scholar] [CrossRef]
- Johnson, S.J.; Bayerl, T.M.; McDermott, D.C.; Adam, G.W.; Rennie, A.R.; Thomas, R.K.; Sackmann, E. Structure of an Adsorbed Dimyristoylphosphatidylcholine Bilayer Measured with Specular Reflection of Neutrons. Biophys. J. 1991, 59, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Naumann, C.; Brumm, T.; Bayerl, T.M. Phase Transition Behavior of Single Phosphatidylcholine Bilayers on a Solid Spherical Support Studied by DSC, NMR and FT-IR. Biophys. J. 1992, 63, 1314–1319. [Google Scholar] [CrossRef] [Green Version]
- Merkel, R.; Sackmann, E.; Evans, E. Molecular Friction and Epitactic Coupling between Monolayers in Supported Bilayers. J. De Phys. 1989, 50, 1535–1555. [Google Scholar] [CrossRef]
- Hinterdorfer, P.; Baber, G.; Tamm, L.K. Reconstitution of Membrane Fusion Sites. A Total Internal Reflection Fluorescence Microscopy Study of Influenza Hemagglutinin-Mediated Membrane Fusion. J. Biol. Chem. 1994, 269, 20360–20368. [Google Scholar] [PubMed]
- Salafsky, J.; Groves, J.T.; Boxer, S.G. Architecture and Function of Membrane Proteins in Planar Supported Bilayers: A Study with Photosynthetic Reaction Centers. Biochemistry 1996, 35, 14773–14781. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, F.F.; Textor, M.; Reviakine, I. Asymmetric Distribution of Phosphatidyl Serine in Supported Phospholipid Bilayers on Titanium Dioxide. Langmuir 2006, 22, 3467–3473. [Google Scholar] [CrossRef] [PubMed]
- Reimhult, E.; Höök, F.; Kasemo, B. Vesicle Adsorption on SiO2 and TiO2: Dependence on Vesicle Size. J. Chem. Phys. 2002, 117, 7401–7404. [Google Scholar] [CrossRef]
- Kasemo, B.; Lausmaa, J. Aspects of Surface Physics on Titanium Implants. Swed. Dent. J. Suppl. 1985, 28, 19–36. [Google Scholar]
- Knoll, W.; Bender, K.; Förch, R.; Frank, C.; Götz, H.; Heibel, C.; Jenkins, T.; Jonas, U.; Kibrom, A.; Kügler, R.; et al. Polymer-Tethered Bimolecular Lipid Membranes. In Polymer Membranes/Biomembranes; Springer: Berlin/Heidelberg, Germany, 2009; pp. 197–233. [Google Scholar]
- Rakhmatullina, E.; Mantion, A.; Bürgi, T.; Malinova, V.; Meier, W. Solid-Supported Amphiphilic Triblock Copolymer Membranes Grafted from Gold Surface. J. Polym. Sci. Part. A Polym. Chem. 2009, 47, 1–13. [Google Scholar] [CrossRef]
- Anderson, T.H.; Min, Y.; Weirich, K.L.; Zeng, H.; Fygenson, D.; Israelachvili, J.N. Formation of Supported Bilayers on Silica Substrates. Langmuir 2009, 25, 6997–7005. [Google Scholar] [CrossRef]
- Chimisso, V.; Maffeis, V.; Hürlimann, D.; Palivan, C.G.; Meier, W. Self-Assembled Polymeric Membranes and Nanoassemblies on Surfaces: Preparation, Characterization.; Current Applications. Macromol. Biosci. 2019, 1900257. [Google Scholar] [CrossRef] [Green Version]
- Piehler, J.; Brecht, A.; Valiokas, R.; Liedberg, B.; Gauglitz, G. A High-Density Poly (Ethylene Glycol) Polymer Brush for Immobilization on Glass-Type Surfaces. Biosens. Bioelectron. 2000, 10, 473–481. [Google Scholar] [CrossRef]
- Sackmann, E.; Tanaka, M. Supported Membranes on Soft Polymer Cushions: Fabrication, Characterization and Applications. Trends Biotechnol. 2000, 18, 58–64. [Google Scholar] [CrossRef]
- Mammen, M.; Choi, S.-K.; Whitesides, G.M. Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew. Chem. Int. Ed. 1998, 37, 2754–2794. [Google Scholar] [CrossRef]
- Atanasov, V.; Knorr, N.; Duran, R.S.; Ingebrandt, S.; Offenhäusser, A.; Knoll, W.; Köper, I. Membrane on a Chip: A Functional Tethered Lipid Bilayer Membrane on Silicon Oxide Surfaces. Biophys. J. 2005, 89, 1780–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atanasov, V.; Atanasova, P.P.; Vockenroth, I.K.; Knorr, N.; Köper, I. A Molecular Toolkit for Highly Insulating Tethered Bilayer Lipid Membranes on Various Substrates. Bioconj. Chem. 2006, 17, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Roskamp, R.F.; Vockenroth, I.K.; Eisenmenger, N.; Braunagel, J.; Koeper, I. Functional Tethered Bilayer Lipid Membranes on Aluminum Oxide. ChemPhysChem 2008, 9, 1920–1924. [Google Scholar] [CrossRef] [PubMed]
- Naumann, R.; Schiller, S.M.; Giess, F.; Grohe, B.; Hartman, K.B.; Kärcher, I.; Köper, I.; Lübben, J.; Vasilev, K.; Knoll, W. Tethered Lipid Bilayers on Ultraflat Gold Surfaces. Langmuir 2003, 19, 5435–5443. [Google Scholar] [CrossRef]
- Junghans, A.; Köper, I. Structural Analysis of Tethered Bilayer Lipid Membranes. Langmuir 2010, 13, 11035–11040. [Google Scholar] [CrossRef]
- Beyer, D.; Elender, G.; Knoll, W.; Kühner, M.; Maus, S.; Ringsdorf, H.; Sackmann, E. Influence of Anchor Lipids on the Homogeneity and Mobility of Lipid Bilayers on Thin Polymer Films. Angew. Chem. Int. Ed. Engl. 1996, 35, 1682–1685. [Google Scholar] [CrossRef]
- Knoll, W.; Frank, C.W.; Heibel, C.; Naumann, R.; Offenhäusser, A.; Rühe, J.; Schmidt, E.K.; Shen, W.W.; Sinner, A. Functional Tethered Lipid Bilayers. Rev. Mol. Biotechnol. 2000, 74, 137–158. [Google Scholar] [CrossRef]
- Jordan, R.; Martin, K.; Räder, H.J.; Unger, K.K. Lipopolymers for Surface Functionalizations. 1. Synthesis and Characterization of Terminal Functionalized Poly (N-Propionylethylenimine) s. Macromolecules 2001, 34, 8858–8865. [Google Scholar] [CrossRef]
- Prucker, O.; Naumann, C.A.; Rühe, J.; Knoll, W.; Frank, C.W. Photochemical Attachment of Polymer Films to Solid Surfaces via Monolayers of Benzophenone Derivatives. J. Am. Chem. Soc. 1999, 121, 8766–8770. [Google Scholar] [CrossRef]
- Naumann, C.A.; Prucker, O.; Lehmann, T.; Rühe, J.; Knoll, W.; Frank, C.W. The Polymer-Supported Phospholipid Bilayer: Tethering as a New Approach to Substrate-Membrane Stabilization. Biomacromolecules 2002, 3, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Watkins, E.B.; El-khouri, R.J.; Miller, C.E.; Seaby, B.G.; Majewski, J.; Marques, C.M.; Kuhl, T.L. Structure and Thermodynamics of Lipid Bilayers on Polyethylene Glycol Cushions: Fact and Fiction of PEG Cushioned Membranes. Langmuir 2011, 27, 13618–13628. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Sackmann, E. Polymer-Supported Membranes as Models of The Cell Surface. Nature 2005, 7059, 656–663. [Google Scholar] [CrossRef]
- Götz, H.; Harth, E.; Schiller, S.M.; Frank, C.W.; Knoll, W.; Hawker, C.J. Synthesis of Lipo-Glycopolymer Amphiphiles By Nitroxide-Mediated Living Free-Radical Polymerization. J. Polym. Sci. Part. A Polym. Chem. 2002, 40, 3379–3391. [Google Scholar] [CrossRef]
- Schiller, S.M.; Naumann, R.; Lovejoy, K.; Kunz, H.; Knoll, W. Archaea Analogue Thiolipids for Tethered Bilayer Lipid Membranes on Ultrasmooth Gold Surfaces. Angew. Chem. Int. Ed. 2003, 42, 208–211. [Google Scholar] [CrossRef]
- Baumgart, T.; Offenhäusser, A. Polysaccharide-Supported Planar Bilayer Lipid Model Membranes. Langmuir 2003, 19, 1730–1737. [Google Scholar] [CrossRef] [Green Version]
- Pavinatto, A.; Pavinatto, F.J.; Barros-Timmons, A. Electrostatic Interactions Are Not Sufficient to Account for Chitosan Bioactivity. ACS Appl. Mater. Interfaces 2010, 2, 246–251. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Jin, G. Chitosan Cushioned Phospholipid Membrane and Its Application in Imaging Ellipsometry Based-Biosensor. Appl. Surf. Sci. 2011, 257, 9407–9413. [Google Scholar] [CrossRef] [Green Version]
- Rehfeldt, F.; Steitz, R.; Armes, S.P.; von Klitzing, R.; Gast, A.P.; Tanaka, M. Reversible Activation of Diblock Copolymer Monolayers at t5he Interface by pH Modulation, 2: Membrane Interactions at The Solid/Liquid Interface. J. Phys. Chem. B 2006, 110, 9177–9182. [Google Scholar] [CrossRef]
- Wong, J.Y.; Majewski, J.; Seitz, M.; Park, C.K.; Israelachvili, J.N.; Smith, G.S. Polymer-Cushioned Bilayers. I. A Structural Study of Various Preparation Methods Using Neutron Reflectometry. Biophys. J. 1999, 77, 1445–1457. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Srinivasan, M.P.; Waring, A.J.; Lehrer, R.I.; Longo, M.L.; Stroeve, P. Supported Lipid Bilayers Lifted from the Substrate by Layer-by-Layer Polyion Cushions on Self-Assembled Monolayers. Colloids Surf. B Biointerfaces 2003, 28, 319–329. [Google Scholar] [CrossRef]
- Ye, Q.; Konradi, R.; Textor, M.; Reimhult, E. Liposomes Tethered to Omega-Functional PEG Brushes and Induced Formation of PEG Brush Supported Planar Lipid Bilayers. Langmuir 2009, 25, 13534–13539. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.J.; Albertorio, F.; Daniel, S.; Cremer, P.S. Double Cushions Preserve Transmembrane Protein Mobility in Supported Bilayer Systems. Langmuir 2008, 24, 6820–6826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poudel, K.R.; Keller, D.J.; Brozik, J.A. Single Particle Tracking Reveals Corralling of a Transmembrane Protein in a Double-Cushioned Lipid Bilayer Assembly. Langmuir 2011, 1, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Albertorio, F.; Diaz, A.J.; Yang, T.; Chapa, V.A.; Kataoka, S.; Castellana, E.T.; Cremer, P.S. Fluid and Air-Stable Lipopolymer Membranes for Biosensor Applications. Langmuir 2005, 21, 7476–7482. [Google Scholar] [CrossRef]
- Goennenwein, S.; Tanaka, M.; Hu, B.; Moroder, L.; Sackmann, E. Functional Incorporation of Integrins into Solid Supported Membranes on Ultrathin Films of Cellulose: Impact on Adhesion. Biophys. J. 2003, 85, 646–655. [Google Scholar] [CrossRef] [Green Version]
- Pompe, T.; Zschoche, S.; Herold, N.; Salchert, K.; Gouzy, M.; Sperling, C.; Werner, C. Maleic Anhydride Copolymers a Versatile Platform for Molecular Biosurface Engineering. Biomacromolecules 2003, 4, 1072–1079. [Google Scholar] [CrossRef]
- Renner, L.; Osaki, T.; Chiantia, S.; Schwille, P.; Pompe, T.; Werner, C. Supported Lipid Bilayers on Spacious and pH-Responsive Polymer Cushions with Varied Hydrophilicity. J. Phys. Chem. B 2008, 112, 6373–6378. [Google Scholar] [CrossRef]
- Renner, L.; Pompe, T.; Lemaitre, R.; Drechsel, D.; Werner, C. Controlled Enhancement of Transmembrane Enzyme Activity in Polymer Cushioned Supported Bilayer Membranes. Soft Matter 2010, 6, 5382–5389. [Google Scholar] [CrossRef]
- Hertrich, S.; Stetter, F.; Rühm, A.; Hugel, T.; Nickel, B. Highly Hydrated Deformable Polyethylene Glycol-Tethered Lipid Bilayers. Langmuir 2014, 30, 9442–9447. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.C.; Juo, J.-Y.; Lin, C.-H.; Liao, Y.-H.; Cheng, C.-Y.; Hsieh, C.-L. Characterization of Single-Protein Dynamics in Polymer-Cushioned Lipid Bilayers Derived from Cell Plasma Membranes. J. Phys. Chem. B 2019, 123, 6492–6504. [Google Scholar] [CrossRef] [PubMed]
- Löfås, S.; Johnsson, B. A Novel Hydrogel Matrix on Gold Surfaces in Surface Plasmon Resonance Sensors for Fast and Efficient Covalent Immobilization of Ligands. J. Chem. Soc. Chem. Commun. 1990, 21, 1526–1528. [Google Scholar] [CrossRef]
- Drews, J. Drug Discovery: A Historical Perspective. Science 2000, 5460, 1960–1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Qi, G.; Xu, X.; Wang, L. Lipid Bilayer Membrane Arrays: Fabrication and Applications. In Future Trends in Biotechnology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 121–152. [Google Scholar]
- McConnell, H.M.; Watts, T.H.; Weis, R.M.; Brian, A.A. Supported Planar Membranes in Studies of Cell-Cell Recognition in the Immune System. Biochim. Et Biophys. Acta 1986, 864, 95–106. [Google Scholar] [CrossRef]
- Alon, R.; Hammer, D.A.; Springer, T.A. Lifetime of the P-Selectin-Carbohydrate Bond and Its Response to Tensile Force in Hydrodynamic Flow. Nature 1995, 374, 539–542. [Google Scholar] [CrossRef]
- Grakoui, A.; Bromley, S.K.; Sumen, C.; Davis, M.M.; Shaw, A.S.; Allen, P.M.; Dustin, M.L. The Immunological Synapse: A Molecular Machine Controlling T Cell Activation. Science 1999, 285, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Groves, J.T.; Mahal, L.K.; Bertozzi, C.R. Control of Cell Adhesion and Growth with Micropatterned Supported Lipid Membranes. Langmuir 2001, 17, 5129–5133. [Google Scholar] [CrossRef]
- Stroumpoulis, D.; Zhang, H.; Rubalcava, L.; Gliem, J.; Tirrell, M. Cell Adhesion and Growth to Peptide-Patterned Supported Lipid Membranes. Langmuir 2007, 23, 3849–3856. [Google Scholar] [CrossRef]
- Shen, Y.-X.; Saboe, P.O.; Sines, I.T.; Erbakan, M.; Kumar, M. Biomimetic Membranes: A Review. J. Membr. Sci. 2014, 454, 359–381. [Google Scholar] [CrossRef]
- Antonietti, M.; Förster, S. Vesicles and Liposomes: A Self-Assembly Principle Beyond Lipids. Adv. Mater. 2003, 15, 1323–1333. [Google Scholar] [CrossRef]
- Discher, B.M.; Won, Y.; Ege, D.S.; Lee, J.C.M.; Bates, F.S.; Discher, D.E.; Hammer, D.A. Polymersomes: Tough Vesicles Made from Diblock Copolymers. Science 1999, 284, 1143–1146. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.C.; Santore, M.; Bates, F.S.; Discher, D.E. From Membranes to Melts, Rouse to Reptation: Diffusion in Polymersome Versus Lipid Bilayers. Macromolecules 2002, 35, 323–326. [Google Scholar] [CrossRef]
- Kumar, M.; Grzelakowski, M.; Zilles, J.; Clark, M.; Meier, W. Highly Permeable Polymeric Membranes Based on the Incorporation of the Functional Water Channel Protein Aquaporin, Z. Proc. Natl. Acad. Sci. USA 2007, 104, 20719–20724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakhmatullina, E.; Meier, W. Solid-Supported Block Copolymer Membranes through Interfacial Adsorption of Charged Block Copolymer Vesicles. Langmuir 2008, 24, 6254–6261. [Google Scholar] [CrossRef]
- Tu, Y.; Song, W.; Ren, T.; Shen, Y.; Chowdhury, R.; Rajapaksha, P.; Culp, T.E.; Samineni, L.; Lang, C.; Thokkadam, A. Rapid Fabrication of Precise High-Throughput Filters from Membrane Protein Nanosheets. Nat. Mater. 2020, 19, 347–354. [Google Scholar] [CrossRef]
- Shen, Y.; Song, W.; Barden, D.R.; Ren, T.; Lang, C.; Feroz, H.; Henderson, C.B.; Saboe, P.O.; Tsai, D.; Yan, H. Achieving High Permeability and Enhanced Selectivity for Angstrom-Scale Separations Using Artificial Water Channel Membranes. Nat. Commun. 2018, 9, 1–11. [Google Scholar]
- Jin, H.; Jiao, F.; Daily, M.D.; Chen, Y.; Yan, F.; Ding, Y.-H.; Zhang, X.; Robertson, E.J.; Baer, M.D.; Chen, C.-L. Highly Stable and Self-Repairing Membrane-Mimetic 2d Nanomaterials Assembled from Lipid-Like Peptoids. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Napoli, A.; Sebök, D.; Senti, A.; Meier, W. Block Copolymer Vesicles. In Block Copolymers in Nanoscience; Wiley-VCH Verlag GmbH&Co. KGaA: Weinheim, Germany, 2008; pp. 39–71. [Google Scholar]
- Richter, R.P.; Brisson, A.R. Following the Formation of Supported Lipid Bilayers on Mica: A Study Combining AFM, QCM-D.; Ellipsometry. Biophys. J. 2005, 88, 3422–3433. [Google Scholar] [CrossRef] [Green Version]
- Palivan, C.G.; Goers, R.; Najer, A.; Zhang, X.; Car, A.; Meier, W. Bioinspired Polymer Vesicles and Membranes for Biological and Medical Applications. Chem. Soc. Rev. 2016, 45, 377–411. [Google Scholar] [CrossRef] [Green Version]
- Tabaei, S.R.; Choi, J.-H.; Zan, G.H.; Zhdanov, V.P.; Cho, N.-J. Solvent-Assisted Lipid Bilayer Formation on Silicon Dioxide and Gold. Langmuir 2014, 30, 10363–10373. [Google Scholar] [CrossRef] [PubMed]
- Tabaei, S.R.; Vafaei, S.; Cho, N.-J. Fabrication of Charged Membranes by the Solvent-Assisted Lipid Bilayer (SALB) Formation Method on SiO2 and Al2O3. Phys. Chem. Chem. Phys. 2015, 17, 11546–11552. [Google Scholar] [CrossRef] [PubMed]
- Ferhan, A.R.; Yoon, B.K.; Park, S.; Sut, T.N.; Chin, H.; Park, J.H.; Jackman, J.A.; Cho, N.-J. Solvent-Assisted Preparation of Supported Lipid Bilayers. Nat. Protoc. 2019, 14, 2091–2118. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.A.; Cho, N.-J. Supported Lipid Bilayer Formation: Beyond Vesicle Fusion. Langmuir 2020, 36, 1387–1400. [Google Scholar] [CrossRef]
- Czernohlavek, C.; Schuster, B. Formation and Characteristics of Mixed Lipid/Polymer Membranes on a Crystalline Surface-Layer Protein Lattice. Biointerphases 2020, 15, 011002. [Google Scholar] [CrossRef] [Green Version]
- Richter, R.; Mukhopadhyay, A.; Brisson, A. Pathways of Lipid Vesicle Deposition on Solid Surfaces: A Combined QCM-D and AFM study. Biophys. J. 2003, 85, 3035–3047. [Google Scholar] [CrossRef] [Green Version]
- Czernohlavek, C.; Schuster, B. Formation of Planar Hybrid Lipid/Polymer Membranes Anchored to an S-Layer Protein Lattice by Vesicle Binding and Rupture. Soft Mater. 2020, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.; Comolli, L.R.; Tscheliessnig, R.; Wang, C.; Nam, K.T.; Hexemer, A.; Siegerist, C.E.; de Yoreo, J.J.; Bertozzi, C.R. Self-Assembly of “S-Bilayers”, a Step toward Expanding the Dimensionality of S-Layer Assemblies. ACS Nano 2013, 7, 4946–4953. [Google Scholar] [CrossRef]
- Schuster, B. S-Layer Protein-Based Biosensors. Biosensors 2018, 8, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, J.; Beales, P.A.; Vanderlick, T.K. Giant Phospholipid/Block Copolymer Hybrid Vesicles: Mixing Behavior and Domain Formation. Langmuir 2011, 27, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Itel, F.; Chami, M.; Najer, A.; Lörcher, S.; Dalin Wu, I.A.D.; Meier, W. Molecular Organization and Dynamics in Polymersome Membranes: A Lateral Diffusion Study. Macromolecules 2014, 47, 7588–7596. [Google Scholar] [CrossRef]
- Schulz, M.; Glatte, D.; Meister, A.; Scholtysek, P.; Kerth, A.; Blume, A.; Bacia, K.; Binder, W.H. Hybrid Lipid/Polymer Giant Unilamellar Vesicles: Effects of Incorporated Biocompatible Pib–Peo Block Copolymers on Vesicle Properties. Soft Matter 2011, 7, 8100–8110. [Google Scholar] [CrossRef]
- Nam, J.; Vanderlick, T.K.; Beales, P.A. Formation and Dissolution of Phospholipid Domains with Varying Textures in Hybrid Lipo-Polymersomes. Soft Matter 2012, 8, 7982–7988. [Google Scholar] [CrossRef]
- Hu, S.-W.; Huang, C.-Y.; Tsao, H.-K.; Sheng, Y.-J. Hybrid Membranes of Lipids and Diblock Copolymers: From Homogeneity to Rafts to Phase Separation. Phys. Rev. E 2019, 99, 012403. [Google Scholar] [CrossRef] [PubMed]
- Amado, E.; Kerth, A.; Blume, A.; Kressler, J. Infrared Reflection Absorption Spectroscopy Coupled with Brewster Angle Microscopy for Studying Interactions of Amphiphilic Triblock Copolymers with Phospholipid Monolayers. Langmuir 2008, 24, 10041–10053. [Google Scholar] [CrossRef] [PubMed]
- Meins, L.; Christophe Schatz, J.-F.; Lecommandoux, S.; Sandre, O. Hybrid Polymer/Lipid Vesicles: State of the Art and Future Perspectives. Mater. Today 2013, 16, 397–402. [Google Scholar] [CrossRef]
- Rao, S.; Prestidge, C.A. Polymer-Lipid Hybrid Systems: Merging the Benefits of Polymeric and Lipid-Based Nanocarriers to Improve Oral Drug Delivery. Expert Opin. Drug Deliv. 2016, 13, 691–707. [Google Scholar] [CrossRef]
- Schulz, M.; Werner, S.; Bacia, K.; Binder, W.H. Controlling Molecular Recognition with Lipid/Polymer Domains in Vesicle Membranes. Angew. Chem. Int. Ed. 2013, 52, 1829–1833. [Google Scholar] [CrossRef]
- Thoma, J.; Belegrinou, S.; Rossbach, P.; Grzelakowski, M.; Katarzyna, K.-T.; Meier, W. Membrane Protein Distribution in Composite Polymer–Lipid Thin Films. Chem. Commun. 2012, 48, 8811–8813. [Google Scholar] [CrossRef]
- Beales, P.A.; Khan, S.; Muench, S.P.; Jeuken, L.J.C. Durable Vesicles for Reconstitution of Membrane Proteins in Biotechnology. Biochem. Soc. Trans. 2017, 45, 15–26. [Google Scholar] [CrossRef]
- Fischlechner, M.; Reibetanz, U.; Zaulig, M.; Enderlein, D.; Romanova, J.; Leporatti, S.; Moya, S.; Donath, E. Fusion of Enveloped Virus Nanoparticles with Polyelectrolyte-Supported Lipid Membranes for the Design of Bio/Nonbio Interfaces. Nano Lett. 2007, 7, 3540–3546. [Google Scholar] [CrossRef] [PubMed]
- Habel, J.; Ogbonna, A.; Larsen, N.; Cherré, S.; Kynde, S.; Midtgaard, S.R.; Kinoshita, K.; Krabbe, S.; Vestergaard Jensen, G.; Søndergaard Hansen, J.; et al. Selecting Analytical Tools for Characterization of Polymersomes in Aqueous Solution. RSC Adv. 2015, 5, 79924–79946. [Google Scholar] [CrossRef] [Green Version]
- Pereira, E.M.A.; Kosaka, P.M.; Rosa, H.; Vieira, D.B.; Kawano, Y.; Petri, D.F.S.; Carmona-Ribeiro, A.M. Hybrid Materials from Intermolecular Associations between Cationic Lipid and Polymers. J. Phys. Chem. B 2008, 112, 9301–9310. [Google Scholar] [CrossRef] [PubMed]
- Morigaki, K.; Kiyosue, K.; Taguchi, T. Micropatterned Composite Membranes of Polymerized and Fluid Lipid Bilayers. Langmuir 2004, 20, 7729–7735. [Google Scholar] [CrossRef] [PubMed]
- Mashaghi, S.; van Oijen, A.M. A Versatile Approach to the Generation of Fluid Supported Lipid Bilayers and Its Applications. Biotechnol. Bioeng. 2014, 111, 2076–2081. [Google Scholar] [CrossRef]
- Kang, M.; Lee, B.; Leal, C. Three-Dimensional Microphase Separation and Synergistic Permeability in Stacked Lipid–Polymer Hybrid Membranes. Chem. Mater. 2017, 29, 9120–9132. [Google Scholar] [CrossRef]
- Yatabe, R.; Noda, J.; Tahara, Y.; Naito, Y.; Ikezaki, H.; Toko, K. Analysis of a Lipid/Polymer Membrane for Bitterness Sensing with a Preconditioning Process. Sensors 2015, 15, 22439–22450. [Google Scholar] [CrossRef] [Green Version]
- Toko, K.; Hara, D.; Tahara, Y.; Yasuura, M.; Ikezaki, H. Relationship between the Amount of Bitter Substances Adsorbed onto Lipid/Polymer Membrane and the Electric Response of Taste Sensors. Sensors 2014, 14, 16274–16286. [Google Scholar] [CrossRef]














© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krywko-Cendrowska, A.; di Leone, S.; Bina, M.; Yorulmaz-Avsar, S.; Palivan, C.G.; Meier, W. Recent Advances in Hybrid Biomimetic Polymer-Based Films: from Assembly to Applications. Polymers 2020, 12, 1003. https://doi.org/10.3390/polym12051003
Krywko-Cendrowska A, di Leone S, Bina M, Yorulmaz-Avsar S, Palivan CG, Meier W. Recent Advances in Hybrid Biomimetic Polymer-Based Films: from Assembly to Applications. Polymers. 2020; 12(5):1003. https://doi.org/10.3390/polym12051003
Chicago/Turabian StyleKrywko-Cendrowska, Agata, Stefano di Leone, Maryame Bina, Saziye Yorulmaz-Avsar, Cornelia G. Palivan, and Wolfgang Meier. 2020. "Recent Advances in Hybrid Biomimetic Polymer-Based Films: from Assembly to Applications" Polymers 12, no. 5: 1003. https://doi.org/10.3390/polym12051003
APA StyleKrywko-Cendrowska, A., di Leone, S., Bina, M., Yorulmaz-Avsar, S., Palivan, C. G., & Meier, W. (2020). Recent Advances in Hybrid Biomimetic Polymer-Based Films: from Assembly to Applications. Polymers, 12(5), 1003. https://doi.org/10.3390/polym12051003