Influence of the Surface Modification of Calcium Carbonate on Polyamide 12 Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Functional Filler Production
2.3. Composite Manufacturing
2.3.1. Preparation of Formulations
2.3.2. Compounding
2.4. Composite Analysis
2.4.1. Scanning Electron Microscopy
2.4.2. Thermogravimetric Analysis
2.4.3. Mechanical Properties
2.4.4. Tensile Test
3. Results and Discussion
3.1. Homogeneity of Filler Distribution
3.1.1. Morphology
3.1.2. Filler Amount Implementation
3.2. Mechanical Properties
3.2.1. Influence on Stiffness/Tensile Modulus
3.2.2. Influence on Ductility and Toughness
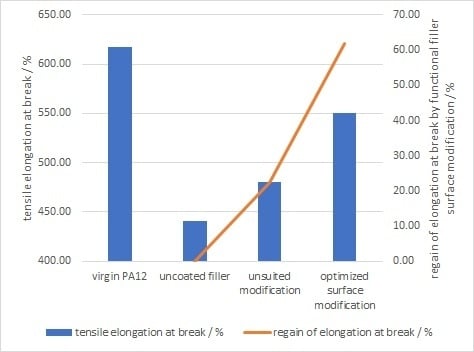
Effect of Surface Modifier Chemistry
Effect of Surface Modifier Amount
4. Conclusions
- The tensile modulus of a compound can be increased by approx. 30 wt % with the introduction of approx. 40 m2 filler per 100 g of PA12.
- This effect on the stiffness is unaffected by the surface modification of the mineral filler, since the tensile modulus is measured before any plastic deformation takes place.
- The ductility as well as tensile strength shows a clear improvement, if the filler is surface modified with an appropriate amino acid instead of stearic acid
- The greatest improvements were obtained with amino acids, which consist of free amino groups at the end of the carboxylic chain, instead of only as side chains.
- To have an optimized filler/coated adhesion promoter ratio, between 1.0 and 1.5 wt % of surface modifier with respect to calcium carbonate is needed. With the tested filler grade, this results in an approx. optimum of 2.5 to 3.0 mmol of treatment agent per 100 m2 CaCO3.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mueller, B. Additive Manufacturing Technologies—Rapid Prototyping to Direct Digital Manufacturing. Assem. Autom. 2012, 32. [Google Scholar] [CrossRef]
- Hague, R.; Mansour, S.; Saleh, N. Design opportunities with rapid manufacturing. Assem. Autom. 2003, 23, 346–356. [Google Scholar] [CrossRef]
- Rambo, C.; Travitzky, N.; Zimmermann, K.; Greil, P. Synthesis of TiC/Ti–Cu composites by pressureless reactive infiltration of TiCu alloy into carbon preforms fabricated by 3D-printing. Mater. Lett. 2005, 59, 1028–1031. [Google Scholar] [CrossRef]
- Murr, L.; Gaytan, S.; Ceylan, A.; Martinez, E.; Martínez, J.; Hernandez, D.; Machado, B.; Ramirez, D.; Medina, F.; Collins, S.; et al. Characterization of titanium aluminide alloy components fabricated by additive manufacturing using electron beam melting. Acta Mater. 2010, 58, 1887–1894. [Google Scholar] [CrossRef]
- Petrovic, V.; Gonzalez, J.V.H.; Ferrando, O.J.; Gordillo, J.D.; Puchades, J.R.B.; Griñan, L.P. Additive layered manufacturing: Sectors of industrial application shown through case studies. Int. J. Prod. Res. 2010, 49, 1061–1079. [Google Scholar] [CrossRef]
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003, 21, 157–161. [Google Scholar] [CrossRef]
- Hofmann, M. 3D Printing Gets a Boost and Opportunities with Polymer Materials. ACS Macro Lett. 2014, 3, 382–386. [Google Scholar] [CrossRef]
- Gill, S.S.; Kaplas, M. Comparative Study of 3D Printing Technologies for Rapid Casting of Aluminium Alloy. Mater. Manuf. Process. 2009, 24, 1405–1411. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing; Springer: New York, NY, USA, 2014. [Google Scholar]
- Liu-Lan, L.; Yu-Sheng, S.; Fan-Di, Z.; Shu-Huai, H. Microstructure of selective laser sintered polyamide. J. Wuhan Univ. Technol. Sci. Ed. 2003, 18, 60–63. [Google Scholar] [CrossRef]
- Childs, T.H.C.; Berzins, M.; Ryder, G.R.; Tontowi, A. Selective laser sintering of an amorphous polymer—simulations and experiments. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 1999, 213, 333–349. [Google Scholar] [CrossRef]
- Schmidt, M.; Pohle, D.; Rechtenwald, T. Selective Laser Sintering of PEEK. CIRP Ann. 2007, 56, 205–208. [Google Scholar] [CrossRef]
- Koo, J.; Lao, S.; Ho, W.; Ngyuen, K.; Cheng, J.; Pilato, L.; Wissler, G.; Ervin, M. Polyamide nanocomposites for selective laser sintering. Proc. SFF Symp. Austin 2006, 392–409. [Google Scholar] [CrossRef]
- Schmid, M.; Amado, A.; Wegener, K. Polymer Powders for Selective Laser Sintering (SLS); ETH-Zürich: Zürich, Switzerland, 2014. [Google Scholar]
- Kim, J.; Creasy, T. Selective laser sintering characteristics of nylon 6/clay-reinforced nanocomposite. Polym. Test. 2004, 23, 629–636. [Google Scholar] [CrossRef]
- Kumar, S. Selective Laser Sintering/Melting, Comprehensive Materials Processing; Hashmi, S., Batalha, G.F., Van Tyne, C.J., Yilbas, B., Eds.; Elsevier: Oxford, UK, 2014; pp. 93–134. [Google Scholar]
- Goodridge, R.; Tuck, C.; Hague, R. Laser sintering of polyamides and other polymers. Prog. Mater. Sci. 2012, 57, 229–267. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, K.; Bourell, D.L. Preparation and laser sintering of limestone PA 12 composite. Polym. Test. 2014, 37, 210–215. [Google Scholar] [CrossRef]
- Sreenivasan, R.; Goel, A.; Bourell, D. Sustainability issues in laser-based additive manufacturing. Phys. Procedia 2010, 5, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Ford, S.; Despeisse, M. Additive manufacturing and sustainability: An exploratory study of the advantages and challenges. J. Clean Prod. 2016, 137, 1573–1587. [Google Scholar] [CrossRef]
- Le Bourhis, F.; Kerbrat, O.; Hascoet, J.-Y.; Mognol, P. Sustainable manufacturing: Evaluation and modeling of environmental impacts in additive manufacturing. Int. J. Adv. Manuf. Technol. 2013, 69, 1927–1939. [Google Scholar] [CrossRef] [Green Version]
- Ippolito, F.; Rentsch, S.; Hübner, G.; Claypole, T.; Gane, P. Influence of calcium carbonate on polyamide 12 regarding melting, formability and crystallization properties. Compos. Part B Eng. 2019, 164, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Goodman, H. Surface-Modified Fillers for Polymer Resins Compositions. International Publication Number WO 2005026252 A1, 24 March 2005. [Google Scholar]
- Shi, X.; Rosa, R.; Lazzeri, A. On the Coating of Precipitated Calcium Carbonate with Stearic Acid in Aqueous Medium. Langmuir 2010, 26, 8474–8482. [Google Scholar] [CrossRef]
- Croitoru, C.; Pascu, A.; Roata, I.C.; Stanciu, E.M. Obtaining and Characterization of Polyolefin-Filled Calcium Carbonate Composites Modified with Stearic Acid; IOP Publishing: Iasi, Rumania, 2017; Volume 209, p. 12041. [Google Scholar]
- Leong, Y.; Abu Bakar, M.B.; Ishak, Z.A.M.; Ariffin, A.; Pukánszky, B. Comparison of the mechanical properties and interfacial interactions between talc, kaolin, and calcium carbonate filled polypropylene composites. J. Appl. Polym. Sci. 2004, 91, 3315–3326. [Google Scholar] [CrossRef]
- Broda, J.; Slusarczyk, C.; Fabia, J.; Demsar, A. Formation and properties of polypropylene/stearic acid composite fibers. Text. Res. J. 2015, 86, 64–71. [Google Scholar] [CrossRef]













| Omyafilm 753–OM | PA2200 | |
|---|---|---|
| producer/supplier | Omya International | EOS e-Manufacturing |
| volume-based median particle size | 2 μm | 60 μm |
| particle shape | irregular | spherical |
| approx. thermal conductivity at 298 K | 1.3 Wm−1 K−1 | 0.2 Wm−1 K−1 |
| approx. specific heat | 0.8 kJkg−1 K−1 | 1.2 kJkg−1 K−1 |
| specific surface area (BET) | 3.7 m2 g−1 |
| Stearic Acid | Amino Hexanoic Acid | ε-Caprolactam | l-Arginine | Glutamic Acid | |
|---|---|---|---|---|---|
| producer/supplier | Wilfar | Sigma Aldrich | Sigma Aldrich | Sigma Aldrich | Sigma Aldrich |
| CAS Number | 57-11-4 | 60-32-2 | 105-60-20 | 74-79-3 | 56-86-0 |
| Linear Formula | C18H36O2 | C6H12NO2 | C6H11NO | C6H14N4O2 | C5H9NO4 |
| Molecular weight | 284.5 g mol−1 | 131.17 g mol−1 | 113.2 g mol−1 | 174.2 g mol−1 | 147.1 g mol−1 |
| Filler Definition | Surface Modifier | Additive Amount/% by Weight (wt %) |
|---|---|---|
| A | Stearic acid | 1.0 ± 0.1 |
| B.1 | Amino hexanoic acid | 0.5 ± 0.1 |
| B.2 | Amino hexanoic acid | 1.0 ± 0.1 |
| B.3 | Amino hexanoic acid | 1.5 ± 0.1 |
| B.4 | Amino hexanoic acid | 2.0 ± 0.1 |
| C | ε-Caprolactam | 1.0 ± 0.1 |
| D | l-Arginine | 1.0 ± 0.1 |
| E | Glutamic acid | 1.0 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ippolito, F.; Hübner, G.; Claypole, T.; Gane, P. Influence of the Surface Modification of Calcium Carbonate on Polyamide 12 Composites. Polymers 2020, 12, 1295. https://doi.org/10.3390/polym12061295
Ippolito F, Hübner G, Claypole T, Gane P. Influence of the Surface Modification of Calcium Carbonate on Polyamide 12 Composites. Polymers. 2020; 12(6):1295. https://doi.org/10.3390/polym12061295
Chicago/Turabian StyleIppolito, Fabio, Gunter Hübner, Tim Claypole, and Patrick Gane. 2020. "Influence of the Surface Modification of Calcium Carbonate on Polyamide 12 Composites" Polymers 12, no. 6: 1295. https://doi.org/10.3390/polym12061295
APA StyleIppolito, F., Hübner, G., Claypole, T., & Gane, P. (2020). Influence of the Surface Modification of Calcium Carbonate on Polyamide 12 Composites. Polymers, 12(6), 1295. https://doi.org/10.3390/polym12061295