Implantation of Recyclability and Healability into Cross-Linked Commercial Polymers by Applying the Vitrimer Concept
Abstract
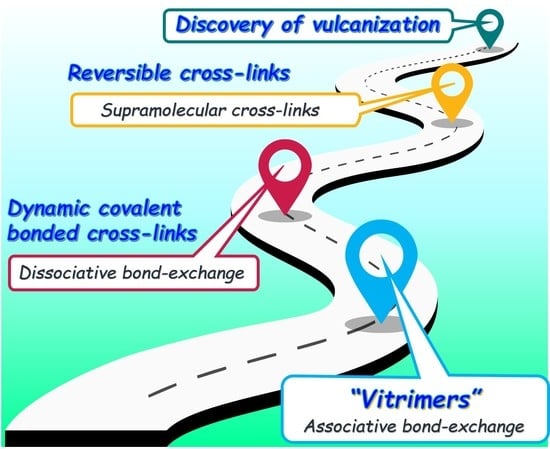
:1. Introduction
2. Characteristics of the Bond Exchange Mechanism of Vitrimers
3. Characteristics of the Physical Properties of Vitrimers
4. Representative Useful Functions of Vitrimers
5. Recent Studies on the Implantation of the Vitrimer Concept into Commercial Polymers
5.1. Polyesters
5.2. Polylactide
5.3. Polycarbonates
5.4. Polydimethylsiloxanes
5.5. Polydienes
5.6. Polyurethanes
5.7. Polyolefins
5.8. Poly(meth)acrylates
5.9. Poly(styrene)
5.10. Other Trends for the Practical Application of the Vitrimer Concept
6. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Himenz, P.C.; Lodge, T.P. Polymer Chemistry, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular polymers. Chem. Rev. 2001, 101, 4071–4097. [Google Scholar] [CrossRef] [PubMed]
- Aida, T.; Meijer, E.W.; Stupp, S.I. Functional Supramolecular Polymers. Science 2012, 335, 813–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, A.; Takashima, Y.; Nakahata, M. Supramolecular Polymeric Materials via Cyclodextrin-Guest Interactions. Acc. Chem. Res. 2014, 47, 2128–2140. [Google Scholar] [CrossRef] [PubMed]
- Noro, A.; Hayashi, M.; Matsushita, Y. Design and properties of supramolecular polymer gels. Soft Matter 2012, 8, 6416–6429. [Google Scholar] [CrossRef]
- Lehn, J.M. Supramolecular Chemistry-Scope and Perspectives Molecules, Supermolecules, and Molecular Devices. Angew. Chem. Int. Ed. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Lehn, J.M. Perspectives in Supramolecular Chemistry—From Molecular Recognition towards Molecular Information Processing and Self-Organization. Angew. Chem. Int. Ed. 1990, 29, 1304–1319. [Google Scholar] [CrossRef]
- Tang, Z.H.; Huang, J.; Guo, B.C.; Zhang, L.Q.; Liu, F. Bioinspired Engineering of Sacrificial Metal-Ligand Bonds into Elastomers with Supramechanical Performance and Adaptive Recovery. Macromolecules 2016, 49, 1781–1789. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Tang, Z.H.; Huang, J.; Guo, B.C.; Huang, G.S. Bioinspired Engineering of Two Different Types of Sacrificial Bonds into Chemically Cross-Linked cis-1,4-Polyisoprene toward a High-Performance Elastomer. Macromolecules 2016, 49, 8593–8604. [Google Scholar] [CrossRef]
- Jiang, F.; Fang, C.; Zhang, J.; Wang, W.T.; Wang, Z.G. Triblock Copolymer Elastomers with Enhanced Mechanical Properties Synthesized by RAFT Polymerization and Subsequent Quaternization through Incorporation of a Comonomer with Imidazole Groups of about 2.0 Mass Percentage. Macromolecules 2017, 50, 6218–6226. [Google Scholar] [CrossRef]
- Hayashi, M.; Matsushima, S.; Noro, A.; Matsushita, Y. Mechanical Property Enhancement of ABA Block Copolymer-Based Elastomers by Incorporating Transient Cross-Links into Soft Middle Block. Macromolecules 2015, 48, 421–431. [Google Scholar] [CrossRef]
- Hayashi, M.; Noro, A.; Matsushita, Y. Highly Extensible Supramolecular Elastomers with Large Stress Generation Capability Originating from Multiple Hydrogen Bonds on the Long Soft Network Strands. Macromol. Rapid Commun. 2016, 37, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Kawana, S.; Nakagawa, S.; Nakai, S.; Sakamoto, M.; Ishii, Y.; Yoshie, N. Interphase synergistic effects of dynamic bonds in multiphase thermoplastic elastomers. J. Mater. Chem. A 2019, 7, 21195–21206. [Google Scholar] [CrossRef]
- Wojtecki, R.J.; Meador, M.A.; Rowan, S.J. Using the dynamic bond to access macroscopically responsive structurally dynamic polymers. Nat. Mater. 2011, 10, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Yu, C.; Denman, R.J.; Zhang, W. Recent advances in dynamic covalent chemistry. Chem. Soc. Rev. 2013, 42, 6634–6654. [Google Scholar] [CrossRef] [PubMed]
- Podgorski, M.; Fairbanks, B.D.; Kirkpatrick, B.E.; McBride, M.; Martinez, A.; Dobson, A.; Bongiardina, N.J.; Bowman, C.N. Toward Stimuli-Responsive Dynamic Thermosets through Continuous Development and Improvements in Covalent Adaptable Networks (CANs). Adv. Mater. 2020, 32, 1906876. [Google Scholar] [CrossRef] [PubMed]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winne, J.M.; Leibler, L.; Du Prez, F.E. Dynamic covalent chemistry in polymer networks: A mechanistic perspective. Polym. Chem. 2019, 10, 6091–6108. [Google Scholar] [CrossRef]
- Scheutz, G.M.; Lessard, J.J.; Sims, M.B.; Sumerlin, B.S. Adaptable Crosslinks in Polymeric Materials: Resolving the Intersection of Thermoplastics and Thermosets. J. Am. Chem. Soc. 2019, 141, 16181–16196. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.B.; Nutt, S.R.; Sheran, K.; Wudl, F. A thermally re-mendable cross-linked polymeric material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H. Reorganization of polymer structures based on dynamic covalent chemistry: Polymer reactions by dynamic covalent exchanges of alkoxyamine units. Polym. J. 2013, 45, 879–891. [Google Scholar] [CrossRef]
- Scott, T.F.; Schneider, A.D.; Cook, W.D.; Bowman, C.N. Photoinduced plasticity in cross-linked polymers. Science 2005, 308, 1615–1617. [Google Scholar] [CrossRef] [PubMed]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-Like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Capelot, M.; Unterlass, M.M.; Tournilhac, F.; Leibler, L. Catalytic Control of the Vitrimer Glass Transition. ACS Macro Lett. 2012, 1, 789–792. [Google Scholar] [CrossRef]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-Catalyzed Transesterification for Healing and Assembling of Thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Yano, R.; Takasu, A. Synthesis of amorphous low T-g polyesters with multiple COOH side groups and their utilization for elastomeric vitrimers based on post-polymerization cross-linking. Polym. Chem. 2019, 10, 2047–2056. [Google Scholar] [CrossRef]
- Hayashi, M.; Yano, R. Fair Investigation of Cross-Link Density Effects on the Bond-Exchange Properties for Trans-Esterification-Based Vitrimers with Identical Concentrations of Reactive Groups. Macromolecules 2020, 53, 182–189. [Google Scholar] [CrossRef]
- Li, L.Q.; Chen, X.; Jin, K.L.; Torkelson, J.M. Vitrimers Designed Both To Strongly Suppress Creep and To Recover Original Cross-Link Density after Reprocessing: Quantitative Theory and Experiments. Macromolecules 2018, 51, 5537–5546. [Google Scholar] [CrossRef]
- Chakma, P.; Digby, Z.A.; Shulman, M.P.; Kuhn, L.R.; Morley, C.N.; Sparks, J.L.; Konkolewicz, D. Anilinium Salts in Polymer Networks for Materials with Mechanical Stability and Mild Thermally Induced Dynamic Properties. ACS Macro Lett. 2019, 8, 95–100. [Google Scholar] [CrossRef]
- Chakma, P.; Morley, C.N.; Sparks, J.L.; Konkolewicz, D. Exploring How Vitrimer-like Properties Can Be Achieved from Dissociative Exchange in Anilinium Salts. Macromolecules 2020, 53, 1233–1244. [Google Scholar] [CrossRef]
- Obadia, M.M.; Mudraboyina, B.P.; Serghei, A.; Montarnal, D.; Drockenmuller, E. Reprocessing and Recycling of Highly Cross-Linked Ion-Conducting Networks through Transalkylation Exchanges of C-N Bonds. J. Am. Chem. Soc. 2015, 137, 6078–6083. [Google Scholar] [CrossRef] [PubMed]
- Obadia, M.M.; Jourdain, A.; Cassagnau, P.; Montarnal, D.; Drockenmuller, E. Tuning the Viscosity Profile of Ionic Vitrimers Incorporating 1,2,3-Triazolium Cross-Links. Adv. Funct. Mater. 2017, 27, 1703258. [Google Scholar] [CrossRef]
- Lopez, G.; Granado, L.; Coqui, G.; Larez-Sosa, A.; Louvain, N.; Ameduri, B. Perfluoropolyether (PFPE)-Based Vitrimers with Ionic Conductivity. Macromolecules 2019, 52, 2148–2155. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.J.; Tang, Z.H.; Wu, S.W.; Guo, B.C. Reprocessable and robust crosslinked elastomers via interfacial C-N transalkylation of pyridinium. Compos. Sci. Technol. 2018, 168, 320–326. [Google Scholar] [CrossRef]
- Hayashi, M.; Chen, L. Functionalization of triblock copolymer elastomers by cross-linking the end blocks via trans-N-alkylation-based exchangeable bonds. Polym. Chem. 2020, 11, 1713–1719. [Google Scholar] [CrossRef]
- Ying, H.Z.; Zhang, Y.F.; Cheng, J.J. Dynamic urea bond for the design of reversible and self-healing polymers. Nat. Commun. 2014, 5, 3218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Ying, H.Z.; Hart, K.R.; Wu, Y.X.; Hsu, A.J.; Coppola, A.M.; Kim, T.A.; Yang, K.; Sottos, N.R.; White, S.R.; et al. Malleable and Recyclable Poly(urea-urethane) Thermosets bearing Hindered Urea Bonds. Adv. Mater. 2016, 28, 7646–7651. [Google Scholar] [CrossRef]
- Zhang, L.H.; Rowan, S.J. Effect of Sterics and Degree of Cross-Linking on the Mechanical Properties of Dynamic Poly(alkylurea-urethane) Networks. Macromolecules 2017, 50, 5051–5060. [Google Scholar] [CrossRef]
- Liu, W.X.; Zhang, C.; Zhang, H.; Zhao, N.; Yu, Z.X.; Xu, J. Oxime-Based and Catalyst-Free Dynamic Covalent Polyurethanes. J. Am. Chem. Soc. 2017, 139, 8678–8684. [Google Scholar] [CrossRef] [PubMed]
- Demongeot, A.; Mougnier, S.J.; Okada, S.; Soulie-Ziakovic, C.; Tournilhac, F. Coordination and catalysis of Zn2+ in epoxy-based vitrimers. Polym. Chem. 2016, 7, 4486–4493. [Google Scholar] [CrossRef]
- Altuna, F.I.; Hoppe, C.E.; Williams, R.J.J. Epoxy Vitrimers: The Effect of Transesterification Reactions on the Network Structure. Polymers 2018, 10, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demongeot, A.; Groote, R.; Goossens, H.; Hoeks, T.; Tournilhac, F.; Leibler, L. Cross-Linking of Poly(butylene terephthalate) by Reactive Extrusion Using Zn(II) Epoxy-Vitrimer Chemistry. Macromolecules 2017, 50, 6117–6127. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.W.; Goossens, J.G.P.; Sijbesma, R.P.; Heuts, J.P.A. Poly(butylene terephthalate)/Glycerol-based Vitrimers via Solid-State Polymerization. Macromolecules 2017, 50, 6742–6751. [Google Scholar] [CrossRef]
- Pei, Z.Q.; Yang, Y.; Chen, Q.M.; Terentjev, E.M.; Wei, Y.; Ji, Y. Mouldable liquid-crystalline elastomer actuators with exchangeable covalent bonds. Nat. Mater. 2014, 13, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Hanzon, D.W.; Traugutt, N.A.; McBride, M.K.; Bowman, C.N.; Yakacki, C.M.; Yu, K. Adaptable liquid crystal elastomers with transesterification-based bond exchange reactions. Soft Matter 2018, 14, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Schmidt, D.F.; Reynaud, E. Catalyst Selection, Creep, and Stress Relaxation in High-Performance Epoxy Vitrimers. Ind. Eng. Chem. Res. 2017, 56, 2667–2672. [Google Scholar] [CrossRef]
- Niu, X.L.; Wang, F.F.; Li, X.H.; Zhang, R.C.; Wu, Q.; Sun, P.C. Using Zn2+ lonomer To Catalyze Transesterification Reaction in Epoxy Vitrimer. Ind. Eng. Chem. Res. 2019, 58, 5698–5706. [Google Scholar] [CrossRef]
- Han, J.R.; Liu, T.; Hao, C.; Zhang, S.; Guo, B.H.; Zhang, J.W. A Catalyst-Free Epoxy Vitrimer System Based on Multifunctional Hyperbranched Polymer. Macromolecules 2018, 51, 6789–6799. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, S.; Hao, C.; Verdi, C.; Liu, W.C.; Liu, H.; Zhang, J.W. Glycerol Induced Catalyst-Free Curing of Epoxy and Vitrimer Preparation. Macromol. Rapid Commun. 2019, 40, 1800889. [Google Scholar] [CrossRef] [PubMed]
- Han, J.R.; Liu, T.; Zhang, S.; Hao, C.; Xin, J.N.; Guo, B.H.; Zhang, J.W. Hyperbranched Polymer Assisted Curing and Repairing of an Epoxy Coating. Ind. Eng. Chem. Res. 2019, 58, 6466–6475. [Google Scholar] [CrossRef]
- Li, Y.Y.; Liu, T.; Zhang, S.; Shao, L.; Fei, M.G.; Yu, H.; Zhang, J.W. Catalyst-free vitrimer elastomers based on a dimer acid: Robust mechanical performance, adaptability and hydrothermal recyclability. Green Chem. 2020, 22, 870–881. [Google Scholar] [CrossRef]
- Altuna, F.I.; Hoppe, C.E.; Williams, R.J.J. Epoxy vitrimers with a covalently bonded tertiary amine as catalyst of the transesterification reaction. Eur. Polym. J. 2019, 113, 297–304. [Google Scholar] [CrossRef]
- Brutman, J.P.; Delgado, P.A.; Hillmyer, M.A. Polylactide Vitrimers. Acs Macro Lett. 2014, 3, 607–610. [Google Scholar] [CrossRef] [Green Version]
- Snyder, R.L.; Fortman, D.J.; De Hoe, G.X.; Hillmyer, M.A.; Dichtel, W.R. Reprocessable Acid-Degradable Polycarbonate Vitrimers. Macromolecules 2018, 51, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Cai, C.; Liu, W.X.; Li, D.D.; Zhang, J.W.; Zhao, N.; Xu, J. Recyclable Polydimethylsiloxane Network Crosslinked by Dynamic Transesterification Reaction. Sci. Rep. 2017, 7, 11833. [Google Scholar] [CrossRef] [PubMed]
- Stukenbroeker, T.; Wang, W.D.; Winne, J.M.; Du Prez, F.E.; Nicolaÿ, R.; Leibler, L. Polydimethylsiloxane quenchable vitrimers. Polym. Chem. 2017, 8, 6590–6593. [Google Scholar] [CrossRef]
- Denissen, W.; Rivero, G.; Nicolaÿ, R.; Leibler, L.; Winne, J.M.; Du Prez, F.E. Vinylogous Urethane Vitrimers. Adv. Funct. Mater. 2015, 25, 2451–2457. [Google Scholar] [CrossRef]
- Ishibashi, J.S.A.; Kalow, J.A. Vitrimeric Silicone Elastomers Enabled by Dynamic Meldrum’s Acid-Derived Cross-Links. ACS Macro Lett. 2018, 7, 482–486. [Google Scholar] [CrossRef]
- Liu, Y.J.; Tang, Z.H.; Wu, S.W.; Guo, B.C. Integrating Sacrificial Bonds into Dynamic Covalent Networks toward Mechanically Robust and Malleable Elastomers. ACS Macro Lett. 2019, 8, 193–199. [Google Scholar] [CrossRef]
- Breuillac, A.; Kassalias, A.; Nicolaÿ, R. Polybutadiene Vitrimers Based on Dioxaborolane Chemistry and Dual Networks with Static and Dynamic Cross-links. Macromolecules 2019, 52, 7102–7113. [Google Scholar] [CrossRef]
- Fortman, D.J.; Brutman, J.P.; Cramer, C.J.; Hillmyer, M.A.; Dichtel, W.R. Mechanically Activated, Catalyst-Free Polyhydroxyurethane Vitrimers. J. Am. Chem. Soc. 2015, 137, 14019–14022. [Google Scholar] [CrossRef] [PubMed]
- Fortman, D.J.; Brutman, J.P.; Hillmyer, M.A.; Dichtel, W.R. Structural effects on the reprocessability and stress relaxation of crosslinked polyhydroxyurethanes. J. Appl. Polym. Sci. 2017, 134, 44984. [Google Scholar] [CrossRef] [Green Version]
- Brutman, J.P.; Fortman, D.J.; De Hoe, G.X.; Dichtel, W.R.; Hillmyer, M.A. Mechanistic Study of Stress Relaxation in Urethane-Containing Polymer Networks. J. Phys. Chem. B. 2019, 123, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Fortman, D.J.; Sheppard, D.T.; Dichtel, W.R. Reprocessing Cross-Linked Polyurethanes by Catalyzing Carbamate Exchange. Macromolecules 2019, 52, 6330–6335. [Google Scholar] [CrossRef]
- Ji, F.C.; Liu, X.D.; Lin, C.H.; Zhou, Y.; Dong, L.; Xu, S.B.; Sheng, D.K.; Yang, Y.M. Reprocessable and Recyclable Crosslinked Polyethylene with Triple Shape Memory Effect. Macromol. Mater. Eng. 2019, 304, 1800528. [Google Scholar] [CrossRef]
- Caffy, F.; Nicolaÿ, R. Transformation of polyethylene into a vitrimer by nitroxide radical coupling of a bis-dioxaborolane. Polym. Chem. 2019, 10, 3107–3115. [Google Scholar] [CrossRef]
- Lessard, J.J.; Garcia, L.F.; Easterling, C.P.; Sims, M.B.; Bentz, K.C.; Arencibia, S.; Savin, D.A.; Sumerlin, B.S. Catalyst-Free Vitrimers from Vinyl Polymers. Macromolecules 2019, 52, 2105–2111. [Google Scholar] [CrossRef]
- Rottger, M.; Domenech, T.; van der Weegen, R.; Breuillac, A.; Nicolaÿ, R.; Leibler, L. High-performance vitrimers from commodity thermoplastics through dioxaborolane metathesis. Science 2017, 356, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Kaushal, S.; Ojha, U. Catalyst-Free Partially Bio-Based Polyester Vitrimers. ACS Appl. Polym. Mater. 2020, 2, 1006–1013. [Google Scholar] [CrossRef]
- Nishimura, Y.; Chung, J.; Muradyan, H.; Guan, Z.B. Silyl Ether as a Robust and Thermally Stable Dynamic Covalent Motif for Malleable Polymer Design. J. Am. Chem. Soc. 2017, 139, 14881–14884. [Google Scholar] [CrossRef] [PubMed]
- Guerre, M.; Taplan, C.; Nicolaÿ, R.; Winne, J.M.; Du Prez, F.E. Fluorinated Vitrimer Elastomers with a Dual Temperature Response. J. Am. Chem. Soc. 2018, 140, 13272–13284. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, X.; Yu, R.; Zhao, X.J.; Zhang, Y.; Huang, W. A facile access to stiff epoxy vitrimers with excellent mechanical properties via siloxane equilibration. J. Mater. Chem. A 2018, 6, 10184–10188. [Google Scholar] [CrossRef]
- Lossada, F.; Jiao, D.J.; Yao, X.Y.; Walther, A. Waterborne Methacrylate-Based Vitrimers. ACS Macro Lett. 2020, 9, 70–76. [Google Scholar] [CrossRef]
- Lessard, J.J.; Scheutz, G.M.; Sung, S.H.; Lantz, K.A.; Epps, T.H.; Sumerlin, B.S. Block Copolymer Vitrimers. J. Am. Chem. Soc. 2020, 142, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, Z.H.; Zhang, X.H.; Liu, Y.J.; Wu, S.W.; Guo, B.C. Covalently Cross-Linked Elastomers with Self-Healing and Malleable Abilities Enabled by Boronic Ester Bonds. ACS Appl. Mater. Interfaces 2018, 10, 24224–24231. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.C.; Yue, L.; Rui, G.C.; Manas-Zloczower, I. Recycling Poly(ethylene-vinyl acetate) with Improved Properties through Dynamic Cross-Linking. Macromolecules 2020, 53, 458–464. [Google Scholar] [CrossRef]
- Ricarte, R.G.; Tournilhac, F.; Leibler, L. Phase Separation and Self-Assembly in Vitrimers: Hierarchical Morphology of Molten and Semicrystalline Polyethylene/Dioxaborolane Maleimide Systems. Macromolecules 2019, 52, 432–443. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.H.; Liu, Y.J.; Guo, B.C.; Zhang, L.Q. Malleable, Mechanically Strong, and Adaptive Elastomers Enabled by Interfacial Exchangeable Bonds. Macromolecules 2017, 50, 7584–7592. [Google Scholar] [CrossRef]
- Qiu, M.; Wu, S.W.; Fang, S.F.; Tang, Z.H.; Guo, B.C. Sustainable, recyclable and robust elastomers enabled by exchangeable interfacial cross-linking. J. Mater. Chem. A 2018, 6, 13607–13612. [Google Scholar] [CrossRef]
- Yang, Z.H.; Wang, Q.H.; Wang, T.M. Dual-Triggered and Thermally Reconfigurable Shape Memory Graphene-Vitrimer Composites. ACS Appl. Mater. Interfaces 2016, 8, 21691–21699. [Google Scholar] [CrossRef] [PubMed]
- Legrand, A.; Soulie-Ziakovic, C. Silica-Epoxy Vitrimer Nanocomposites. Macromolecules 2016, 49, 5893–5902. [Google Scholar] [CrossRef]
- Liu, Y.J.; Tang, Z.H.; Chen, Y.; Zhang, C.F.; Guo, B.C. Engineering of beta-Hydroxyl Esters into Elastomer-Nanoparticle Interface toward Malleable, Robust, and Reprocessable Vitrimer Composites. ACS Appl. Mater. Interfaces 2018, 10, 2992–3001. [Google Scholar] [CrossRef] [PubMed]
- Spiesschaert, Y.; Guerre, M.; Imbernon, L.; Winne, J.M.; Du Prez, F. Filler reinforced polydimethylsiloxane-based vitrimers. Polymer 2019, 172, 239–246. [Google Scholar] [CrossRef]
- Wang, Z.H.; Li, Z.; Wei, Y.; Ji, Y. Gold Nanospheres Dispersed Light Responsive Epoxy Vitrimers. Polymers 2018, 10, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lossada, F.; Guo, J.Q.; Jiao, D.J.; Groeer, S.; Bourgeat-Lami, E.; Montarnal, D.; Walther, A. Vitrimer Chemistry Meets Cellulose Nanofibrils: Bioinspired Nanopapers with High Water Resistance and Strong Adhesion. Biomacromolecules 2019, 20, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.B.; Ma, S.Q.; Yan, S.F.; Zhu, J. Readily recyclable carbon fiber reinforced composites based on degradable thermosets: A review. Green Chem. 2019, 21, 5781–5796. [Google Scholar] [CrossRef]
- Denissen, W.; De Baere, I.; Van Paepegem, W.; Leibler, L.; Winne, J.; Du Prez, F.E. Vinylogous Urea Vitrimers and Their Application in Fiber Reinforced Composites. Macromolecules 2018, 51, 2054–2064. [Google Scholar] [CrossRef] [Green Version]
- Chabert, E.; Vial, J.; Cauchois, J.P.; Mihaluta, M.; Tournilhac, F. Multiple welding of long fiber epoxy vitrimer composites. Soft Matter 2016, 12, 4838–4845. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Y.; Wang, Z.H.; Zhang, X.Y.; Chen, Q.M.; Qian, X.J.; Liu, N.; Wei, Y.; Ji, Y. Polydopamine nanoparticles doped in liquid crystal elastomers for producing dynamic 3D structures. J. Mater. Chem. A 2017, 5, 6740–6746. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, F.S.; Li, Z.; Qiao, J.; Wei, Y.; Ji, Y. Enabling the sunlight driven response of thermally induced shape memory polymers by rewritable CH3NH3PbI3 perovskite coating. J. Mater. Chem. A 2017, 5, 7285–7290. [Google Scholar] [CrossRef]
- Yang, Y.; Pei, Z.Q.; Zhang, X.Q.; Tao, L.; Wei, Y.; Ji, Y. Carbon nanotube-vitrimer composite for facile and efficient photo-welding of epoxy. Chem. Sci. 2014, 5, 3486–3492. [Google Scholar] [CrossRef]
- Cash, J.J.; Kubo, T.; Dobbins, D.J.; Sumerlin, B.S. Maximizing the symbiosis of static and dynamic bonds in self-healing boronic ester networks. Polym. Chem. 2018, 9, 2011–2020. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, L.; Wu, Y.P.; Zhao, X.L.; Zhang, Y.J. Rapid Stress Relaxation and Moderate Temperature of Malleability Enabled by the Synergy of Disulfide Metathesis and Carboxylate Transesterification in Epoxy Vitrimers. ACS Macro Lett. 2019, 8, 255–260. [Google Scholar] [CrossRef]
- Hamel, C.M.; Kuang, X.; Chen, K.J.; Qi, H.J. Reaction-Diffusion Model for Thermosetting Polymer Dissolution through Exchange Reactions Assisted by Small-Molecule Solvents. Macromolecules 2019, 52, 3636–3645. [Google Scholar] [CrossRef]
- Shi, Q.; Yu, K.; Dunn, M.L.; Wang, T.J.; Qi, H.J. Solvent Assisted Pressure-Free Surface Welding and Reprocessing of Malleable Epoxy Polymers. Macromolecules 2016, 49, 5527–5537. [Google Scholar] [CrossRef]
- Kuang, X.; Shi, Q.; Zhou, Y.Y.; Zhao, Z.; Wang, T.J.; Qi, H.J. Dissolution of epoxy thermosets via mild alcoholysis: The mechanism and kinetics study. RSC Adv. 2018, 8, 1493–1502. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.J.; Luo, C.Q.; Lu, H.B.; Yu, K. Primary recycling of anhydride-cured engineering epoxy using alcohol solvent. Polym. Eng. Sci. 2019, 59, E111–E119. [Google Scholar] [CrossRef]
- Liu, T.; Hao, C.; Wang, L.W.; Li, Y.Z.; Liu, W.C.; Xin, J.N.; Zhang, J.W. Eugenol-Derived Biobased Epoxy: Shape Memory, Repairing, and Recyclability. Macromolecules 2017, 50, 8588–8597. [Google Scholar] [CrossRef]
- Memon, H.; Liu, H.Y.; Rashid, M.A.; Chen, L.; Jiang, Q.R.; Zhang, L.Y.; Wei, Y.; Liu, W.S.; Qiu, Y.P. Vanillin-Based Epoxy Vitrimer with High Performance and Closed-Loop Recyclability. Macromolecules 2020, 53, 621–630. [Google Scholar] [CrossRef]
- Altuna, F.I.; Pettarin, V.; Williams, R.J.J. Self-healable polymer networks based on the cross-linking of epoxidised soybean oil by an aqueous citric acid solution. Green Chem. 2013, 15, 3360–3366. [Google Scholar] [CrossRef]
- Yu, K.; Taynton, P.; Zhang, W.; Dunn, M.L.; Qi, H.J. Influence of stoichiometry on the glass transition and bond exchange reactions in epoxy thermoset polymers. RSC Adv. 2014, 4, 48682–48690. [Google Scholar] [CrossRef]
- Zhao, S.; Abu-Omar, M.M. Catechol-Mediated Glycidylation toward Epoxy Vitrimers/Polymers with Tunable Properties. Macromolecules 2019, 52, 3646–3654. [Google Scholar] [CrossRef]
- Miao, W.S.; Zou, W.K.; Luo, Y.W.; Zheng, N.; Zhao, Q.; Xie, T. Structural tuning of polycaprolactone based thermadapt shape memory polymer. Polym. Chem. 2020, 11, 1369–1374. [Google Scholar] [CrossRef]
- Wu, S.L.; Yang, H.H.; Huang, S.Y.; Chen, Q. Relationship between Reaction Kinetics and Chain Dynamics of Vitrimers Based on Dioxaborolane Metathesis. Macromolecules 2020, 53, 1180–1190. [Google Scholar] [CrossRef]
- Ricarte, R.G.; Tournilhac, F.; Cloitre, M.; Leibler, L. Linear Viscoelasticity and Flow of Self-Assembled Vitrimers: The Case of a Polyethylene/Dioxaborolane System. Macromolecules 2020, 53, 1852–1866. [Google Scholar] [CrossRef]
- Wu, J.B.; Li, S.J.; Liu, H.; Qian, H.J.; Lu, Z.Y. Dynamics and reaction kinetics of coarse-grained bulk vitrimers: A molecular dynamics study. Phys. Chem. Chem. Phys. 2019, 21, 13258–13267. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, A.; Asbai, R.; Anaya, O.; Chehimi, M.M.; Drockenmuller, E.; Montarnal, D. Rheological Properties of Covalent Adaptable Networks with 1,2,3-Triazolium Cross-Links: The Missing Link between Vitrimers and Dissociative Networks. Macromolecules 2020, 53, 1884–1900. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Kong, S.X.; Tang, Z.H.; Wu, S.W.; Guo, B.C.; Zhang, L.Q. Facile Strategy for the Biomimetic Heterogeneous Design of Elastomers with Mechanical Robustness, Malleability, and Functionality. ACS Macro Lett. 2020, 9, 49–55. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, W.F.; Zhu, S.P. Reversible Shape Memory Polymer from Semicrystalline Poly(ethylene-co-vinyl acetate) with Dynamic Covalent Polymer Networks. Macromolecules 2018, 51, 8956–8963. [Google Scholar] [CrossRef]
- Pei, Z.Q.; Yang, Y.; Chen, Q.M.; Wei, Y.; Ji, Y. Regional Shape Control of Strategically Assembled Multishape Memory Vitrimers. Adv. Mater. 2016, 28, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.M.; Yu, X.W.; Pei, Z.Q.; Yang, Y.; Wei, Y.; Ji, Y. Multi-stimuli responsive and multi-functional oligoaniline-modified vitrimers. Chem. Sci. 2017, 8, 724–733. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.Z.; Zheng, N.; Zhao, Q.; Xie, T. Healable, Reconfigurable, Reprocessable Thermoset Shape Memory Polymer with Highly Tunable Topological Rearrangement Kinetics. ACS Appl. Mater. Interfaces 2017, 9, 22077–22082. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Y.; Wang, Y.; Zhu, J.; Yu, J.R.; Hu, Z.M. Bio-Based Epoxy Vitrimers: Reprocessibility, Controllable Shape Memory, and Degradability. J. Polym. Sci. Pol. Chem. 2017, 55, 1790–1799. [Google Scholar] [CrossRef]
- Shi, Q.; Yu, K.; Kuang, X.; Mu, X.M.; Dunn, C.K.; Dunn, M.L.; Wang, T.J.; Qi, H.J. Recyclable 3D printing of vitrimer epoxy. Mater. Horiz. 2017, 4, 598–607. [Google Scholar] [CrossRef]
- Zhang, B.; Kowsari, K.; Serjouei, A.; Dunn, M.L.; Ge, Q. Reprocessable thermosets for sustainable three-dimensional printing. Nat. Commun. 2018, 9, 1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
















© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, M. Implantation of Recyclability and Healability into Cross-Linked Commercial Polymers by Applying the Vitrimer Concept. Polymers 2020, 12, 1322. https://doi.org/10.3390/polym12061322
Hayashi M. Implantation of Recyclability and Healability into Cross-Linked Commercial Polymers by Applying the Vitrimer Concept. Polymers. 2020; 12(6):1322. https://doi.org/10.3390/polym12061322
Chicago/Turabian StyleHayashi, Mikihiro. 2020. "Implantation of Recyclability and Healability into Cross-Linked Commercial Polymers by Applying the Vitrimer Concept" Polymers 12, no. 6: 1322. https://doi.org/10.3390/polym12061322
APA StyleHayashi, M. (2020). Implantation of Recyclability and Healability into Cross-Linked Commercial Polymers by Applying the Vitrimer Concept. Polymers, 12(6), 1322. https://doi.org/10.3390/polym12061322