Encapsulation of Bioactive Compounds from Aloe Vera Agrowastes in Electrospun Poly (Ethylene Oxide) Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Aloe Vera Extract
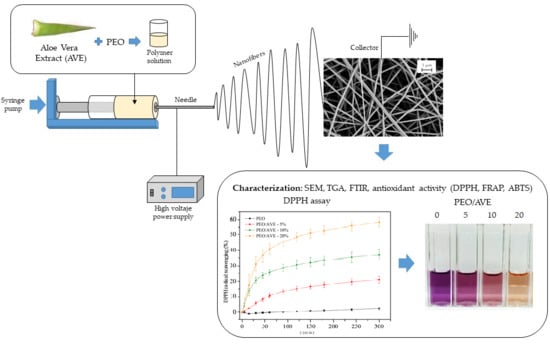
2.3. Preparation of Electrospun Nanofibers
2.4. Characterization of Electrospun Nanofibers
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.3. Thermal Analysis
2.4.4. Antioxidant Activity
DPPH Radical Scavenging Method
FRAP Method
ABTS Radical Scavenging Method
2.4.5. Encapsulation Efficiency
2.5. Statistical Analysis
3. Results
3.1. SEM
3.2. FTIR
3.3. Thermogravimetric Analysis
3.4. Antioxidant Activity and Encapsulation Efficiency
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana-Méridas, O.; González-Coloma, A.; Sánchez-Vioque, R. Agricultural residues as a source of bioactive natural products. Phytochem. Rev. 2012, 11, 447–466. [Google Scholar] [CrossRef]
- Fritsch, C.; Staebler, A.; Happel, A.; Márquez, M.A.C.; Aguiló-Aguayo, I.; Abadias, M.; Gallur, M.; Cigognini, I.M.; Montanari, A.; López, M.J.; et al. Processing, valorization and application of bio-waste derived compounds from potato, tomato, olive and cereals: A review. Sustainability 2017, 9, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuck, C.O.; Perez, E.; Horvath, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Flores-López, M.L.; Romaní, A.; Cerqueira, M.A.; Rodríguez-García, R.; Jasso de Rodríguez, D.; Vicente, A.A. Compositional features and bioactive properties of whole fraction from Aloe vera processing. Ind. Crops Prod. 2016, 91, 179–185. [Google Scholar] [CrossRef] [Green Version]
- López, A.; Suárez de Tangil, M.; Vega-Orellana, O.; Ramírez, A.S.; Rico, M. Phenolic Constituents, Antioxidant and Preliminary Antimycoplasmic Activities of Leaf Skin and Flowers of Aloe vera (L.) Burm. f. (syn. A. barbadensis Mill.) from the Canary Islands (Spain). Molecules 2013, 18, 4942–4954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-Assisted Extraction of Phenolic Compounds from Almond Skin Byproducts (Prunus amygdalus): A Multivariate Analysis Approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef] [Green Version]
- Bouras, M.; Chadni, M.; Barba, F.J.; Grimi, N.; Bals, O.; Vorobiev, E. Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Ind. Crops Prod. 2015, 77, 590–601. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Mellinas, C.; Garrigós, M.C.; Balart, R.; Torres-Giner, S. Optimization of Microwave-Assisted Extraction of Phenolic Compounds with Antioxidant Activity from Carob Pods. Food Anal. Methods 2019, 12, 2480–2490. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Barreiro, M.F.; Carvalho, A.M.; Oliveira, M.B.P.P.; Curran, T.P.; Ferreira, I.C.F.R. Valorisation of tomato wastes for development of nutrient-rich antioxidant ingredients: A sustainable approach towards the needs of the today’s society. Innov. Food Sci. Emerg. Technol. 2017, 41, 160–171. [Google Scholar] [CrossRef] [Green Version]
- Cacciotti, I.; Mori, S.; Cherubini, V.; Nanni, F. Eco-sustainable systems based on poly(lactic acid), diatomite and coffee grounds extract for food packaging. Int. J. Biol. Macromol. 2018, 112, 567–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgos, N.; Valdés, A.; Jiménez, A. Valorization of Agricultural Wastes for the Production of Protein-Based Biopolymers. J. Renew. Mater. 2016, 4, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Valdés, A.; Mellinas, C.; Ramos, M.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Use of herbs, spices and their bioactive compounds in active food packaging. RSC Adv. 2015, 5, 40324–40335. [Google Scholar] [CrossRef] [Green Version]
- Valdés, A.; Mellinas, C.; Ramos, M.; Garrigós, M.C.; Jiménez, A. Natural additives and agricultural wastes in biopolymer formulations for food packaging. Front. Chem. 2014, 2, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; Sendón, R.; Sanches-Silva, A. Aloe vera: Ancient knowledge with new frontiers. Trends Food Sci. Technol. 2017, 61, 94–102. [Google Scholar] [CrossRef]
- Baruah, A.; Bordoloi, M.; Deka Baruah, H.P. Aloe vera: A multipurpose industrial crop. Ind. Crops Prod. 2016, 94, 951–963. [Google Scholar] [CrossRef]
- Hamman, J.H. Composition and applications of Aloe vera leaf gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, T.; Dweck, A.C. Aloe vera leaf gel: A review update. J. Ethnopharmacol. 1999, 68, 3–37. [Google Scholar] [CrossRef]
- Eshun, K.; He, Q. Aloe Vera: A Valuable Ingredient for the Food, Pharmaceutical and Cosmetic Industries—A Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, E.; Darias-Martín, J.; Díaz-Romero, C. Aloe vera as a Functional Ingredient in Foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 305–326. [Google Scholar] [CrossRef] [PubMed]
- Lucini, L.; Pellizzoni, M.; Pellegrino, R.; Molinari, G.P.; Colla, G. Phytochemical constituents and in vitro radical scavenging activity of different Aloe species. Food Chem. 2015, 170, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Quispe, C.; Villalobos, M.; Bórquez, J.; Simirgiotis, M. Chemical Composition and Antioxidant Activity of Aloe vera from the Pica Oasis (Tarapacá, Chile) by UHPLC-Q/Orbitrap/MS/MS. J. Chem. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-Compound-Extraction Systems for Fruit and Vegetable Samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef]
- Talmaciu, A.I.; Volf, I.; Popa, V.I. A Comparative Analysis of the ‘Green’ Techniques Applied for Polyphenols Extraction from Bioresources. Chem. Biodivers. 2015, 12, 1635–1651. [Google Scholar] [CrossRef] [PubMed]
- Mandal, V.; Tandey, R. A critical analysis of publication trends from 2005-2015 in microwave assisted extraction of botanicals: How far we have come and the road ahead. TrAC Trends Anal. Chem. 2016, 82, 100–108. [Google Scholar] [CrossRef]
- Kala, H.K.; Mehta, R.; Sen, K.K.; Tandey, R.; Mandal, V. Critical analysis of research trends and issues in microwave assisted extraction of phenolics: Have we really done enough. TrAC Trends Anal. Chem. 2016, 85, 140–152. [Google Scholar] [CrossRef]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C.; Kung, F.W.L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, L.; Del Nobile, M.A.; Conte, A. The extraction of bioactive compounds from food residues using microwaves. Curr. Opin. Food Sci. 2015, 5, 93–98. [Google Scholar] [CrossRef]
- Khoshnoudi-Nia, S.; Sharif, N.; Jafari, S.M. Loading of phenolic compounds into electrospun nanofibers and electrosprayed nanoparticles. Trends Food Sci. Technol. 2020, 95, 59–74. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Assadpour, E.; Mahdi Jafari, S. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef] [PubMed]
- Vilchez, A.; Acevedo, F.; Cea, M.; Seeger, M.; Navia, R. Applications of electrospun nanofibers with antioxidant properties: A review. Nanomaterials 2020, 10, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Drosou, C.G.; Krokida, M.K.; Biliaderis, C.G. Encapsulation of bioactive compounds through electrospinning/electrospraying and spray drying: A comparative assessment of food-related applications. Dry. Technol. 2017, 35, 139–162. [Google Scholar] [CrossRef]
- Wen, P.; Zong, M.-H.; Linhardt, R.J.; Feng, K.; Wu, H. Electrospinning: A novel nano-encapsulation approach for bioactive compounds. Trends Food Sci. Technol. 2017, 70, 56–68. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. Advances in Spray-Drying Encapsulation of Food Bioactive Ingredients: From Microcapsules to Nanocapsules. Annu. Rev. Food Sci. Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Haider, S.; Kang, I.K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2015, 11, 1165–1188. [Google Scholar] [CrossRef]
- Bianco, A.; di Federico, E.; Cacciotti, I. Electrospun poly(ε-caprolactone)-based composites using synthesized β-tricalcium phosphate. Polym. Adv. Technol. 2011, 22, 1832–1841. [Google Scholar] [CrossRef]
- Cacciotti, I.; Ciocci, M.; Di Giovanni, E.; Nanni, F.; Melino, S. Hydrogen sulfide-releasing fibrous membranes: Potential patches for stimulating human stem cells proliferation and viability under oxidative stress. Int. J. Mol. Sci. 2018, 19, 2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacciotti, I.; Chronopoulou, L.; Palocci, C.; Amalfitano, A.; Cantiani, M.; Cordaro, M.; Lajolo, C.; Callà, C.; Boninsegna, A.; Lucchetti, D.; et al. Controlled release of 18-β-glycyrrhetic acid by nanodelivery system increases cytotoxicity on oral carcinoma cell line. Nanotechnology 2018, 29, 285101. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I.; Pallotto, F.; Scognamiglio, V.; Moscone, D.; Arduini, F. Reusable optical multi-plate sensing system for pesticide detection by using electrospun membranes as smart support for acetylcholinesterase immobilisation. Mater. Sci. Eng. C 2020, 111, 110744. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Kriegel, C.; Arrechi, A.; Kit, K.; McClements, D.J.; Weiss, J. Fabrication, functionalization, and application of electrospun biopolymer nanofibers. Crit. Rev. Food Sci. Nutr. 2008, 48, 775–797. [Google Scholar] [CrossRef]
- Anu Bhushani, J.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Cacciotti, I.; Fortunati, E.; Puglia, D.; Kenny, J.M.; Nanni, F. Effect of silver nanoparticles and cellulose nanocrystals on electrospun poly(lactic) acid mats: Morphology, thermal properties and mechanical behavior. Carbohydr. Polym. 2014, 103, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacciotti, I.; Nanni, F. Poly(lactic) acid fibers loaded with mesoporous silica for potential applications in the active food packaging. AIP Conf. Proc. 2016, 1738. [Google Scholar]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer (Guildf) 2008, 49, 2387–2425. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Estevez-Areco, S.; Guz, L.; Candal, R.; Goyanes, S. Release kinetics of rosemary (Rosmarinus officinalis) polyphenols from polyvinyl alcohol (PVA) electrospun nanofibers in several food simulants. Food Packag. Shelf Life 2018, 18, 42–50. [Google Scholar] [CrossRef]
- Vatankhah, E. Rosmarinic acid-loaded electrospun nanofibers: In vitro release kinetic study and bioactivity assessment. Eng. Life Sci. 2018, 18, 732–742. [Google Scholar] [CrossRef] [Green Version]
- Locilento, D.A.; Mercante, L.A.; Andre, R.S.; Mattoso, L.H.C.; Luna, G.L.F.; Brassolatti, P.; de Anibal, F.F.; Correa, D.S. Biocompatible and biodegradable electrospun nanofibrous membranes loaded with grape seed extract for wound dressing application. J. Nanomater. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Faki, R.; Gursoy, O.; Yilmaz, Y. Effect of Electrospinning Process on Total Antioxidant Activity of Electrospun Nanofibers Containing Grape Seed Extract. Open Chem. 2019, 17, 912–918. [Google Scholar] [CrossRef]
- Lin, S.; Chen, M.; Jiang, H.; Fan, L.; Sun, B.; Yu, F.; Yang, X.; Lou, X.; He, C.; Wang, H. Green electrospun grape seed extract-loaded silk fibroin nanofibrous mats with excellent cytocompatibility and antioxidant effect. Colloids Surf. B Biointerfaces 2016, 139, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Torkamani, A.E.; Syahariza, Z.A.; Norziah, M.H.; Wan, A.K.M.; Juliano, P. Encapsulation of polyphenolic antioxidants obtained from Momordica charantia fruit within zein/gelatin shell core fibers via coaxial electrospinning. Food Biosci. 2018, 21, 60–71. [Google Scholar] [CrossRef]
- Maver, T.; Kurečič, M.; Pivec, T.; Maver, U.; Gradišnik, L.; Gašparič, P.; Kaker, B.; Bratuša, A.; Hribernik, S.; Stana Kleinschek, K. Needleless electrospun carboxymethyl cellulose/polyethylene oxide mats with medicinal plant extracts for advanced wound care applications. Cellulose 2020, 1–22. [Google Scholar] [CrossRef]
- He, L.; Lan, W.; Ahmed, S.; Qin, W.; Liu, Y. Electrospun polyvinyl alcohol film containing pomegranate peel extract and sodium dehydroacetate for use as food packaging. Food Packag. Shelf Life 2019, 22, 100390. [Google Scholar] [CrossRef]
- Bruni, G.P.; dos Acunha, T.S.; Oliveira, J.P.; Fonseca, L.M.; Silva, F.T.; Guimarães, V.M.; Zavareze, E. da R. Electrospun protein fibers loaded with yerba-mate extract for bioactive release in food packaging. J. Sci. Food Agric. 2020, 100, 3341–3350. [Google Scholar] [CrossRef]
- Bootdee, K.; Nithitanakul, M. Poly(d,l-lactide-co-glycolide) nanospheres within composite poly(vinyl alcohol)/aloe vera electrospun nanofiber as a novel wound dressing for controlled release of drug. Int. J. Polym. Mater. Polym. Biomater. 2019, 0, 1–8. [Google Scholar] [CrossRef]
- Ezhilarasu, H.; Ramalingam, R.; Dhand, C.; Lakshminarayanan, R.; Sadiq, A.; Gandhimathi, C.; Ramakrishna, S.; Bay, B.H.; Venugopal, J.R.; Srinivasan, D.K. Biocompatible Aloe vera and Tetracycline Hydrochloride Loaded Hybrid Nanofibrous Scaffolds for Skin Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 5174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baghersad, S.; Hajir Bahrami, S.; Mohammadi, M.R.; Mojtahedi, M.R.M.; Milan, P.B. Development of biodegradable electrospun gelatin/aloe-vera/poly(ε-caprolactone) hybrid nanofibrous scaffold for application as skin substitutes. Mater. Sci. Eng. C 2018, 93, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Nezhad-Mokhtari, P.; Ramazani, S. Aloe vera-loaded nanofibrous scaffold based on Zein/Polycaprolactone/Collagen for wound healing. Int. J. Biol. Macromol. 2020, 153, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Aghamohamadi, N.; Sanjani, N.S.; Majidi, R.F.; Nasrollahi, S.A. Preparation and characterization of Aloe vera acetate and electrospinning fibers as promising antibacterial properties materials. Mater. Sci. Eng. C 2019, 94, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Orue, I.; Gainza, G.; Garcia-Garcia, P.; Gutierrez, F.B.; Aguirre, J.J.; Hernandez, R.M.; Delgado, A.; Igartua, M. Composite nanofibrous membranes of PLGA/Aloe vera containing lipid nanoparticles for wound dressing applications. Int. J. Pharm. 2019, 556, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Pathalamuthu, P.; Siddharthan, A.; Giridev, V.R.; Victoria, V.; Thangam, R.; Sivasubramanian, S.; Savariar, V.; Hemamalini, T. Enhanced performance of Aloe vera incorporated chitosan-polyethylene oxide electrospun wound scaffold produced using novel Spirograph based collector assembly. Int. J. Biol. Macromol. 2019, 140, 808–824. [Google Scholar] [CrossRef] [PubMed]
- Torres-Giner, S.; Wilkanowicz, S.; Melendez-Rodriguez, B.; Lagaron, J.M. Nanoencapsulation of Aloe vera in Synthetic and Naturally Occurring Polymers by Electrohydrodynamic Processing of Interest in Food Technology and Bioactive Packaging. J. Agric. Food Chem. 2017, 65, 4439–4448. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Dai, Y.; Cui, H. Antibacterial poly(ethylene oxide) electrospun nanofibers containing cinnamon essential oil/beta-cyclodextrin proteoliposomes. Carbohydr. Polym. 2017, 178, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cui, H.; Li, C.; Lin, L. A novel polyethylene oxide/Dendrobium officinale nanofiber: Preparation, characterization and application in pork packaging. Food Packag. Shelf Life 2019, 21, 100329. [Google Scholar] [CrossRef]
- Aydogdu, A.; Yildiz, E.; Aydogdu, Y.; Sumnu, G.; Sahin, S.; Ayhan, Z. Enhancing oxidative stability of walnuts by using gallic acid loaded lentil flour based electrospun nanofibers as active packaging material. Food Hydrocoll. 2019, 95, 245–255. [Google Scholar] [CrossRef]
- Cui, H.; Wu, J.; Li, C.; Lin, L. Improving anti-listeria activity of cheese packaging via nanofiber containing nisin-loaded nanoparticles. LWT Food Sci. Technol. 2017, 81, 233–242. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Lin, L. Plasma-treated poly(ethylene oxide) nanofibers containing tea tree oil/beta-cyclodextrin inclusion complex for antibacterial packaging. Carbohydr. Polym. 2018, 179, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Keskin, N.O.S.; Tekinay, T.; Uyar, T. Antioxidant α-tocopherol/γ-cyclodextrin-inclusion complex encapsulated poly(lactic acid) electrospun nanofibrous web for food packaging. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, A.; Calderone, M.; Cacciotti, I. Electrospun PHBV/PEO co-solution blends: Microstructure, thermal and mechanical properties. Mater. Sci. Eng. C 2013, 33, 1067–1077. [Google Scholar] [CrossRef]
- Cacciotti, I.; Calderone, M.; Bianco, A. Tailoring the properties of electrospun PHBV mats: Co-solution blending and selective removal of PEO. Eur. Polym. J. 2013, 49, 3210–3222. [Google Scholar] [CrossRef]
- Hossain, M.F.; Gong, R.H.; Rigout, M. Effect of polymer concentration on electrospinning of hydroxypropyl-β-cyclodextrins/PEO nanofibres. J. Text. Inst. 2016, 107, 1511–1518. [Google Scholar] [CrossRef]
- Pakravan, M.; Heuzey, M.C.; Ajji, A. A fundamental study of chitosan/PEO electrospinning. Polymer (Guildf) 2011, 52, 4813–4824. [Google Scholar] [CrossRef]
- Uyar, T.; Hacaloglu, J.; Besenbacher, F. Electrospun Polyethylene Oxide (PEO) Nanofibers Containing Cyclodextrin Inclusion Complex. J. Nanosci. Nanotechnol. 2011, 11, 3949–3958. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Fujihara, K.; Teo, W.E.; Lim, C.T.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005. [Google Scholar]
- Bianco, A.; Cacciotti, L.; Fragalá, M.E.; Lamastra, F.R.; Speghini, A.; Piccinelli, F.; Malandrino, G.; Gusmano, G. Eu-doped titania nanofibers: Processing, thermal behaviour and luminescent properties. J. Nanosci. Nanotechnol. 2010, 10, 5183–5190. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zou, L.; Lu, H.; Wei, Y.; Hua, J.; Chen, S. Preparation and characterization of electrospun PHBV/PEO mats: The role of solvent and PEO component. J. Mater. Sci. 2016, 51, 5695–5711. [Google Scholar] [CrossRef]
- Theron, S.A.; Zussman, E.; Yarin, A.L. Experimental investigation of the governing parameters in the electrospinning of polyethylene oxide solution. Polymer (Guildf) 2004, 45, 2017–2030. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Colín-Orozco, J.; Zapata-Torres, M.; Rodríguez-Gattorno, G.; Pedroza-Islas, R. Properties of Poly (ethylene oxide)/ whey Protein Isolate Nanofibers Prepared by Electrospinning. Food Biophys. 2015, 10, 134–144. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Y.; Li, C.; Liu, L.; Surendhiran, D.; Cui, H. Antibacterial activity of PEO nanofibers incorporating polysaccharide from dandelion and its derivative. Carbohydr. Polym. 2018, 198, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Sukma, M.; Opanasopit, P. Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int. J. Pharm. 2013, 452, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Cardarelli, M.; Rouphael, Y.; Pellizzoni, M.; Colla, G.; Lucini, L. Profile of bioactive secondary metabolites and antioxidant capacity of leaf exudates from eighteen Aloe species. Ind. Crops Prod. 2017, 108, 44–51. [Google Scholar] [CrossRef]
- Lai, Q.; Wang, H.; Guo, X.; Abbasi, A.M.; Wang, T.; Li, T.; Fu, X.; Li, J.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of seven cultivars of Aloe. Int. J. Food Sci. Technol. 2016, 51, 1489–1494. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Xu, Z.; Wu, M.; Xia, W.; Zhang, W. Chimonanthus praecox extract/cyclodextrin inclusion complexes: Selective inclusion, enhancement of antioxidant activity and thermal stability. Ind. Crops Prod. 2017, 95, 60–65. [Google Scholar] [CrossRef]
- Añibarro-Ortega, M.; Pinela, J.; Barros, L.; Ćirić, A.; Silva, S.P.; Coelho, E.; Mocan, A.; Calhelha, R.C.; Soković, M.; Coimbra, M.A.; et al. Compositional features and bioactive properties of aloe vera leaf (Fillet, mucilage, and rind) and flower. Antioxidants 2019, 8, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gülçin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz- Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Kim, S.; Asnin, L.; Assefa, A.D.; Ko, E.Y.; Sharma, K.; Park, S.W. Extraction of Antioxidants from Aloe vera Leaf Gel: A Response Surface Methodology Study. Food Anal. Methods 2014, 7, 1804–1815. [Google Scholar] [CrossRef]
- Ranic, M.; Nikolic, M.; Pavlovic, M.; Buntic, A.; Siler-Marinkovic, S.; Dimitrijevic-Brankovic, S. Optimization of microwave-assisted extraction of natural antioxidants from spent espresso coffee grounds by response surface methodology. J. Clean. Prod. 2014, 80, 69–79. [Google Scholar] [CrossRef]
- Quiroz, J.Q.; Torres, A.C.; Ramirez, L.M.; Garcia, M.S.; Gomez, G.C.; Rojas, J. Optimization of the Microwave-Assisted Extraction Process of Bioactive Compounds from Annatto Seeds (Bixa orellana L.). Antioxidants 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aytac, Z.; Kusku, S.I.; Durgun, E.; Uyar, T. Encapsulation of gallic acid/cyclodextrin inclusion complex in electrospun polylactic acid nanofibers: Release behavior and antioxidant activity of gallic acid. Mater. Sci. Eng. C 2016, 63, 231–239. [Google Scholar] [CrossRef]
- Aytac, Z.; Ipek, S.; Durgun, E.; Tekinay, T.; Uyar, T. Antibacterial electrospun zein nanofibrous web encapsulating thymol/cyclodextrin-inclusion complex for food packaging. Food Chem. 2017, 233, 117–124. [Google Scholar] [CrossRef]
- Yildiz, Z.I.; Celebioglu, A.; Kilic, M.E.; Durgun, E.; Uyar, T. Fast-dissolving carvacrol/cyclodextrin inclusion complex electrospun fibers with enhanced thermal stability, water solubility, and antioxidant activity. J. Mater. Sci. 2018, 53, 15837–15849. [Google Scholar] [CrossRef] [Green Version]
- Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Thymol/cyclodextrin inclusion complex nanofibrous webs: Enhanced water solubility, high thermal stability and antioxidant property of thymol. Food Res. Int. 2018, 106, 280–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, M.; Beltrán, A.; Peltzer, M.; Valente, A.J.M.; del Garrigós, M.C. Release and antioxidant activity of carvacrol and thymol from polypropylene active packaging films. LWT Food Sci. Technol. 2014, 58, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Ciannamea, E.M.; Stefani, P.M.; Ruseckaite, R.A. Properties and antioxidant activity of soy protein concentrate films incorporated with red grape extract processed by casting and compression molding. LWT Food Sci. Technol. 2016, 74, 353–362. [Google Scholar] [CrossRef]
- López De Dicastillo, C.; Nerín, C.; Alfaro, P.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Development of new antioxidant active packaging films based on ethylene vinyl alcohol copolymer (EVOH) and green tea extract. J. Agric. Food Chem. Am. Chem. Soc. 2011, 59, 7832–7840. [Google Scholar] [CrossRef] [PubMed]
- Mellinas, C.; Jiménez, A.; Garrigós, M.C. Microwave-Assisted Green Synthesis and Antioxidant Activity of Selenium Nanoparticles Using Theobroma Cacao L. Bean Shell Extract. Molecules 2019, 24, 4048. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, T.; Bilal, M.; Iqbal, H.M.N.; Li, C. Green biosynthesis of silver nanoparticles using leaves extract of Artemisia vulgaris and their potential biomedical applications. Colloids Surfaces B Biointerfaces 2017, 158, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Nejatzadeh-Barandozi, F. Antibacterial activities and antioxidant capacity of Aloe vera. Org. Med. Chem. Lett. 2013, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Xue, J.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S. Liposome as a Delivery System for Carotenoids: Comparative Antioxidant Activity of Carotenoids As Measured by Ferric Reducing Antioxidant Power, DPPH Assay and Lipid Peroxidation. J. Agric. Food Chem. 2014, 62, 6726–6735. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Xie, J.; Zhang, X.; Cai, J.; Xia, S. Polysaccharide-based nanoparticles by chitosan and gum arabic polyelectrolyte complexation as carriers for curcumin. Food Hydrocoll. 2016, 57, 236–245. [Google Scholar] [CrossRef]
- Aydogdu, A.; Sumnu, G.; Sahin, S. Fabrication of gallic acid loaded Hydroxypropyl methylcellulose nanofibers by electrospinning technique as active packaging material. Carbohydr. Polym. 2019, 208, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Ipek, S.; Durgun, E.; Uyar, T. Antioxidant electrospun zein nanofibrous web encapsulating quercetin/cyclodextrin inclusion complex. J. Mater. Sci. 2018, 53, 1527–1539. [Google Scholar] [CrossRef] [Green Version]
- Celebioglu, A.; Uyar, T. Antioxidant Vitamin E/Cyclodextrin Inclusion Complex Electrospun Nanofibers: Enhanced Water Solubility, Prolonged Shelf Life, and Photostability of Vitamin E. J. Agric. Food Chem. 2017, 65, 5404–5412. [Google Scholar] [CrossRef]
- Li, J.H.; Miao, J.; Wu, J.L.; Chen, S.F.; Zhang, Q.Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- De Araújo, G.K.P.; De Souza, S.J.; Da Silva, M.V.; Yamashita, F.; Gonçalves, O.H.; Leimann, F.V.; Shirai, M.A. Physical, antimicrobial and antioxidant properties of starch-based film containing ethanolic propolis extract. Int. J. Food Sci. Technol. 2015, 50, 2080–2087. [Google Scholar] [CrossRef]
- De Freitas, T.B.; Santos, C.H.K.; da Silva, M.V.; Shirai, M.A.; Dias, M.I.; Barros, L.; Barreiro, M.F.; Ferreira, I.C.F.R.; Gonçalves, O.H.; Leimann, F.V. Antioxidants extraction from Pinhão (Araucaria angustifolia (Bertol.) Kuntze) coats and application to zein films. Food Packag. Shelf Life 2018, 15, 28–34. [Google Scholar] [CrossRef]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernandez-Lopez, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of antibacterial and antioxidant properties of chitosan edible films incorporated with maqui berry (Aristotelia chilensis). LWT Food Sci. Technol. 2015, 64, 1057–1062. [Google Scholar] [CrossRef]
- Aceituno-Medina, M.; Mendoza, S.; Rodríguez, B.A.; Lagaron, J.M.; López-Rubio, A. Improved antioxidant capacity of quercetin and ferulic acid during In-Vitro digestion through encapsulation within food-grade electrospun fibers. J. Funct. Foods 2015, 12, 332–341. [Google Scholar] [CrossRef] [Green Version]
- Amna, T.; Gharsan, F.N.; Shang, K.; Hassan, M.S.; Khil, M.S.; Hwang, I. Electrospun Twin Fibers Encumbered with Intrinsic Antioxidant Activity as Prospective Bandage. Macromol. Res. 2019, 27, 663–669. [Google Scholar] [CrossRef]
- Sarkar, R.; Ghosh, A.; Barui, A.; Datta, P. Repositing honey incorporated electrospun nanofiber membranes to provide anti-oxidant, anti-bacterial and anti-inflammatory microenvironment for wound regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 31. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Nahvi, Z.; Zandi, M. Antioxidant peptide-loaded electrospun chitosan/poly(vinyl alcohol) nanofibrous mat intended for food biopackaging purposes. Food Hydrocoll. 2019, 89, 637–648. [Google Scholar] [CrossRef]








| Sample | T1% (°C) | * TMAX (°C) | * %RES800 °C |
|---|---|---|---|
| PEO | 339.0 | 399.6 ± 1.0 a | 2.0 ± 0.8 a |
| PEO/AVE 5% | 268.3 | 405.9 ± 0.2 b | 5.9 ± 0.4 b |
| PEO/AVE 10% | 204.8 | 408.6 ± 0.0 c | 7.0 ± 0.9 b,c |
| PEO/AVE 20% | 153.8 | 408.5 ± 0.5 c | 9.8 ± 0.6 c |
| Assay | Sample | mAVE,f (mg) | EE (%) | µmolTrolox/mgfiber |
|---|---|---|---|---|
| DPPH | PEO | - | - | - |
| PEO/AVE 5% | 0.046 ± 0.009 a | 92.0 ± 18.1 a | 0.019 ± 0.002 a | |
| PEO/AVE 10% | 0.087 ± 0.014 b | 87.3 ± 13.7 a | 0.029 ± 0.003 b | |
| PEO/AVE 20% | 0.174 ± 0.012 c | 87.2 ± 6.2 a | 0.051 ± 0.003 c | |
| FRAP | PEO | - | - | - |
| PEO/AVE 5% | 0.038 ± 0.002 a | 75.8 ± 4.0 a | 0.028 ± 0.001 a | |
| PEO/AVE 10% | 0.066 ± 0.002 b | 65.9 ± 2.5 a | 0.046 ± 0.002 b | |
| PEO/AVE 20% | 0.130 ± 0.014 c | 65.1 ± 7.1 a | 0.086 ± 0.009 c | |
| ABTS | PEO | - | - | - |
| PEO/AVE 5% | 0.053 ± 0.004 a | 105.2 ± 8.4 a | 0.039 ± 0.002 a | |
| PEO/AVE 10% | 0.094 ± 0.003 b | 93.8 ± 3.0 a | 0.062 ± 0.002 b | |
| PEO/AVE 20% | 0.139 ± 0.009 c | 69.5 ± 4.4 b | 0.087 ± 0.005 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solaberrieta, I.; Jiménez, A.; Cacciotti, I.; Garrigós, M.C. Encapsulation of Bioactive Compounds from Aloe Vera Agrowastes in Electrospun Poly (Ethylene Oxide) Nanofibers. Polymers 2020, 12, 1323. https://doi.org/10.3390/polym12061323
Solaberrieta I, Jiménez A, Cacciotti I, Garrigós MC. Encapsulation of Bioactive Compounds from Aloe Vera Agrowastes in Electrospun Poly (Ethylene Oxide) Nanofibers. Polymers. 2020; 12(6):1323. https://doi.org/10.3390/polym12061323
Chicago/Turabian StyleSolaberrieta, Ignacio, Alfonso Jiménez, Ilaria Cacciotti, and Maria Carmen Garrigós. 2020. "Encapsulation of Bioactive Compounds from Aloe Vera Agrowastes in Electrospun Poly (Ethylene Oxide) Nanofibers" Polymers 12, no. 6: 1323. https://doi.org/10.3390/polym12061323
APA StyleSolaberrieta, I., Jiménez, A., Cacciotti, I., & Garrigós, M. C. (2020). Encapsulation of Bioactive Compounds from Aloe Vera Agrowastes in Electrospun Poly (Ethylene Oxide) Nanofibers. Polymers, 12(6), 1323. https://doi.org/10.3390/polym12061323