Preparation of Electrosprayed, Microporous Particle Filled Layers
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of PTFE Microporous Layers
2.3. Characterization of PTFE Microporous Layers
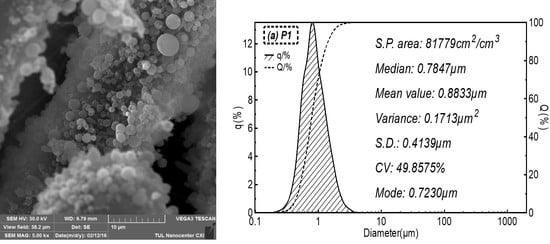
2.3.1. Morphologies
2.3.2. Roughness of PTFE Microporous Layers
2.3.3. Contact Angle Analysis
2.3.4. Electrical Resistance
3. Results and Discussions
3.1. Morphology of Microporous Layers
3.2. Roughness of PTFE Microporous Layers
3.3. Influence on Hydrophobicity
3.4. Influence of Electrical Property on Microporous Layers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bodnár, E.; Grifoll, J.; Rosell-Llompart, J. Polymer solution electrospraying: A tool for engineering particles and films with controlled morphology. J. Aerosol Sci. 2018, 125, 93–118. [Google Scholar] [CrossRef]
- Butt, H.J.; Graf, K.; Kappl, M. Thin films on surfaces of liquids. In Physics and Chemistry of Interfaces; Wiley: Hoboken, NJ, USA, 2003; pp. 280–298. [Google Scholar]
- Yuan, X.D.; Yang, X.J. A study on friction and wear properties of PTFE coatings under vacuum conditions. Wear 2010, 269, 291–297. [Google Scholar] [CrossRef]
- Light, D.N.; Wilcox, J.R. Process Considerations in the Fabrication of Fluoropolymer Printed Circuit Boards. IEEE Trans. Compon. Packag. Manuf. Technol. Part A 1995, 1, 118–126. [Google Scholar] [CrossRef]
- Zhao-Zhu, Z.; Wei-Min, L. Study on the friction and wear properties of glass fabric composites filled with nano- and micro-particles under different conditions. Mater. Sci. Eng. A 2005, 392, 359–365. [Google Scholar]
- Huang, F.; Wei, Q.; Liu, Y.; Gao, W.; Huang, Y. Surface functionalization of silk fabric by PTFE sputter coating. J. Mater. Sci. 2007, 42, 8025–8028. [Google Scholar] [CrossRef]
- Nomura, M.; Tobita, H.; Suzuki, K. Emulsion polymerization: Kinetic and mechanistic aspects. Adv. Polym. Sci. 2005, 175, 1–128. [Google Scholar]
- Manziek, L.; Langenmayr, E.; Lamola, A.; Gallagher, M.; Brese, N.; Annan, N. Functionalized Emulsion and Suspension Polymer Particles: Nanoreactors for the Synthesis of Inorganic Materials. Chem. Mater. 1998, 10, 3101–3108. [Google Scholar] [CrossRef]
- Kasai, H.; Nalwa, H.S.; Oikawa, H.; Okada, S.; Matsuda, H.; Minami, N.; Kakuta, A.; Ono, K.; Mukoh, A.; Nakanishi, H. A novel preparation method of organic microcrystals. Jpn. J. Appl. Phys. 1992, 31, L1132–L1134. [Google Scholar] [CrossRef]
- Mu, L.; Feng, S.S. Fabrication, characterization and in vitro release of paclitaxel (Taxol®) loaded poly (lactic-co-glycolic acid) microspheres prepared by spray drying technique with lipid/cholesterol emulsifiers. J. Control. Release 2001, 76, 239–254. [Google Scholar] [CrossRef]
- Yeo, S.D.; Kiran, E. Formation of polymer particles with supercritical fluids: A review. J. Supercrit. Fluid. 2005, 34, 287–308. [Google Scholar] [CrossRef]
- Dhanumalayan, E.; Joshi, G.M. Performance properties and applications of polytetrafluoroethylene (PTFE)—A review. Adv. Compos. Hybrid. Mater. 2018, 1, 247–268. [Google Scholar] [CrossRef]
- Isık, T.; Demir, M.M.; Aydogan, C.; Ciftci, M.; Yagci, Y. Hydrophobic coatings from photochemically prepared hydrophilic polymethacrylates via electrospraying. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1338–1344. [Google Scholar] [CrossRef]
- Meiron, T.S.; Marmur, A.; Saguy, I.S. Contact angle measurement on rough surfaces. J. Colloid. 2004, 274, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.M.; Hohman, M.M.; Brenner, M.P.; Rutledge, G.C. Experimental characterization of electrospinning: The electri-cally forced jet and instabilities. Polymer 2001, 42, 09955–09967. [Google Scholar] [CrossRef]
- Yarin, A.L.; Koombhongse, S.; Reneker, D.H. Bending instability in electrospinning of nanofibers. J. Appl. Phys. 2001, 89, 3018–3026. [Google Scholar] [CrossRef] [Green Version]
- Long, Q.; Cai, M.; Li, J.; Rong, H.; Jiang, L. Improving the electrical catalytic activity of Pt/TiO2 nanocomposites by a combination of electrospinning and microwave irradiation. J. Nanopart. Res. 2010, 13, 1655–1662. [Google Scholar] [CrossRef]
- Venkataraman, M.; Mishra, R.; Marek, J.; Kucerova, K.; Militky, J. Aerogel embedded electrospun nanofiber layers for thermal insulation. In Textile Bioengineering and Informatics Symposium Proceedings, Proceedings of the 10th Textile Bioengineering and Informatics Symposium, (TBIS 2017), Wuhan, China, 16—19 May 2017; Wuhan Textile University: Wuhan, China, 2017; pp. 745–750. [Google Scholar]
- Burkarter, E.; Saul, C.K.; Thomazi, F.; Cruz, N.C.; Zanata, S.M.; Roman, L.S.; Schreiner, W.H. Electrosprayed superhydrophobic PTFE: A non-contaminating surface. J. Phys. D Appl. Phys. 2007, 40, 7778–7781. [Google Scholar] [CrossRef]
- Venkataraman, M.; Mishra, R.; Subramaniam, V.; Gnanamani, A.; Kotresh, T.M.; Militky, J. Dynamic heat flux measurement for advanced insulation materials. Fibers Polym. 2016, 17, 925–931. [Google Scholar] [CrossRef]
- Burkarter, E.; Saul, C.K.; Thomazi, F.; Cruz, N.C.; Roman, L.S.; Schreiner, W.H. Superhydrophobic electrosprayed PTFE. Surf. Coat. Technol. 2007, 202, 194–198. [Google Scholar] [CrossRef]
- Venkataraman, M.; Mishra, R.; Yang, K.; Militky, J.; Kremenakova, D.; Zhu, G.; Yao, J. Preparation of electrosprayed microporous membranes. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 18th World Textile Conference (AUTEX 2018), Istanbul, Turkey, 20–22 June 2018; IOP Publishing: Bristol, UK, 2018; Volume 460. [Google Scholar]
- Venkataraman, M.; Mishra, R.; Militky, J.; Xiong, X.; Marek, J.; Yao, J.; Zhu, G. Electrospun nanofibrous membranes embedded with aerogel for advanced thermal and transport properties. Polym. Adv. Technol. 2018, 29, 2583–2592. [Google Scholar] [CrossRef]
- Sukigara, S.; Gandhi, M.; Ayutsede, J.; Micklus, M.; Ko, F. Regeneration of Bombyxmori silk by electrospinning—Part 1: Processing parameters and geometric properties. Polymer 2003, 44, 5721. [Google Scholar] [CrossRef]
- Ahn, Y.C.; Park, S.K.; Kim, G.T.; Hwang, Y.J.; Lee, C.G.; Shin, H.S.; Lee, J.K. Development of high efficiency nanofilters made of nanofibers. Curr. Appl. Phys. 2006, 6, 1030. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded nanofibers formed during electrospinning. Polymer. 1999, 40, 4585. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, H.Y.; Bang, H.J.; Jung, Y.H.; Lee, S.G. The change of bead morphology formed on electrospun polystyrene fibers. Polymer. 2003, 44, 4029. [Google Scholar] [CrossRef]
- Lin, K.; Chua, K.N.; Christopherson, G.T.; Lim, S.; Mao, H.Q. Reducing electrospun nanofiber diameter and variability using cationicamphiphiles. Polymer 2007, 48, 6384. [Google Scholar] [CrossRef]
- Jaworek, A. Electrospray droplet sources for thin film deposition. J. Mater. Sci. 2007, 266–297. [Google Scholar] [CrossRef]
- Venkataraman, M.; Mishra, R.; Militky, J.; Wiener, J.; Kucerova, K.; Marek, J.; Arumugam, V. Optimization of microporous hydrophobic membranes by electrospraying. In Proceedings of the 9th International Conference on Nanomaterials—Research and Application, (NANOCON 2017), Brno, Czech Republic, 18–20 October 2017; Volume 2017, pp. 877–8859. [Google Scholar]











| Sample No. | PTFE | Carbon Microparticle | Water & Surfactant |
|---|---|---|---|
| S1 | 60 | - | 40 |
| S2 | 60 | 0.04 | 40 |
| S3 | 55 | - | 45 |
| S4 | 55 | 0.04 | 45 |
| Substrate Speed (mm/min) | Voltage (kV) | Speed of Electrode (rev/min) | In | Tent | Air | |||
|---|---|---|---|---|---|---|---|---|
| RH (%) | T (°C) | RH (%) | T (°C) | RH (%) | T (°C) | |||
| Static | 10/30 | 5.0 | 42 | 22.3 | 46.8 | 22.8 | 49.9 | 22.4 |
| No. | ||||
|---|---|---|---|---|
| P1 | 6.817 ± 2.700 | 8.679 ± 3.071 | −0.213 ± 0.898 | 4.038 ± 0.995 |
| P2 | 7.715 ± 1.212 | 9.820 ± 1.240 | −0.9218 ± 0.233 | 4.108 ± 0.973 |
| P3 | 6.690 ± 2.122 | 8.207 ± 2.479 | −0.577 ± 0.285 | 3.154 ± 1.021 |
| P4 | 14.617 ± 2.453 | 17.032 ± 2.495 | 0.074 ± 0.282 | 1.989 ± 0.289 |
| No. | t (mm) | ||
|---|---|---|---|
| P1 | 2.6924 ± 0.3692 | 4.2960 ± 0.3901 | 0.34 |
| P2 | 3.7044 ± 0.8918 | 8.3430 ± 0.9417 | 0.28 |
| P3 | 3.4196 ± 0.5939 | 7.6942 ± 0.5509 | 0.26 |
| P4 | 2.1392 ± 0.0642 | 4.8132 ± 0.2878 | 0.31 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkataraman, M.; Yang, K.; Xiong, X.; Militky, J.; Kremenakova, D.; Zhu, G.; Yao, J.; Wang, Y.; Zhang, G. Preparation of Electrosprayed, Microporous Particle Filled Layers. Polymers 2020, 12, 1352. https://doi.org/10.3390/polym12061352
Venkataraman M, Yang K, Xiong X, Militky J, Kremenakova D, Zhu G, Yao J, Wang Y, Zhang G. Preparation of Electrosprayed, Microporous Particle Filled Layers. Polymers. 2020; 12(6):1352. https://doi.org/10.3390/polym12061352
Chicago/Turabian StyleVenkataraman, Mohanapriya, Kai Yang, Xiaoman Xiong, Jiri Militky, Dana Kremenakova, Guocheng Zhu, Juming Yao, Yan Wang, and Guoqing Zhang. 2020. "Preparation of Electrosprayed, Microporous Particle Filled Layers" Polymers 12, no. 6: 1352. https://doi.org/10.3390/polym12061352
APA StyleVenkataraman, M., Yang, K., Xiong, X., Militky, J., Kremenakova, D., Zhu, G., Yao, J., Wang, Y., & Zhang, G. (2020). Preparation of Electrosprayed, Microporous Particle Filled Layers. Polymers, 12(6), 1352. https://doi.org/10.3390/polym12061352