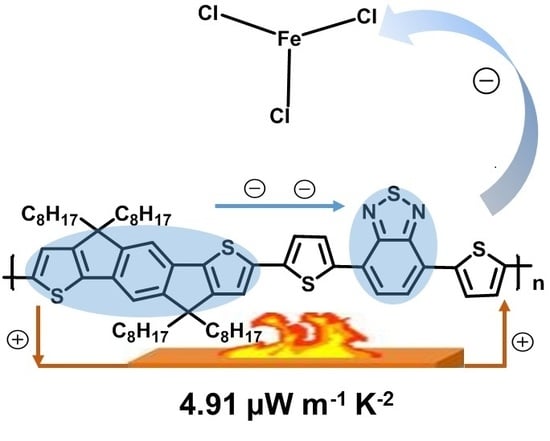
Structure and Doping Optimization of IDT-Based Copolymers for Thermoelectrics
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Polymers
2.2.1. General Synthetic Procedure
2.2.2. 2-Methyl-7-(5-methylthiophen-2-yl)-4,4,9,9-tetraoctyl-4,9-dihydro-s-indaceno[1,2-b:5,6-b’]dithiophene (P1)
2.2.3. 2-Methyl-7-(5’-methyl-[2,2’-dithiophen]-5-yl)-4,4,9,9-tetraoctyl-4,9-dihydro-s-indaceno[1,2-b:5,6-b’]dithiophene (P2)
2.2.4. 4-(5-(7-methyl-4,4,9,9-tetraoctyl-4,9-dihydro-s-indaceno[1,2-b:5,6-b’]dithiophen-2-yl)thiophen-2-yl)-7-(5-methylthiophen-2-yl)benzo[c][1,2,5]thiadiazole (P3)
2.3. Preparation of Polymer Films
2.4. Doping Experiment
3. Results and Discussion
3.1. Optical and Electrochemical Characteristics
3.2. Thin-Film Microstructure
3.3. Thermoelectric Performance
3.4. Photoelectron Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, F.; Zang, Y.; Huang, D.; Di, C.A.; Zhu, D. Flexible and self-powered temperature-pressure dual-parameter sensors using microstructure-frame-supported organic thermoelectric materials. Nat. Commun. 2015, 6, 8356. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, F.; Liu, C.; Wang, W.; Li, X.; Wang, T.; Xu, J. A simple thermoelectric device based on inorganic/organic composite thin film for energy harvesting. Chem. Eng. J. 2017, 320, 201–210. [Google Scholar] [CrossRef]
- Liang, L.; Gao, C.; Chen, G.; Guo, C.-Y. Large-area, stretchable, super flexible and mechanically stable thermoelectric films of polymer/carbon nanotube composites. J. Mater. Chem. C 2016, 4, 526–532. [Google Scholar] [CrossRef]
- Poehler, T.; Katz, H. Prospects for polymer-based thermoelectrics: State of the art and theoretical analysis. Energy Environ. Sci. 2012, 5, 8110. [Google Scholar] [CrossRef]
- Chen, G.; Xu, W.; Zhu, D. Recent advances in organic polymer thermoelectric composites. J. Mater. Chem. C 2017, 5, 4350–4360. [Google Scholar] [CrossRef]
- Bubnova, O.; Crispin, X. Towards poly mer-based organic thermoelectric generators. Energy Environ. Sci. 2012, 5, 9345. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Sun, Y.; Xu, W.; Zhu, D. Organic thermoelectric materials: Emerging green energy materials converting heat to electricity directly and efficiently. Adv. Mater. 2014, 26, 6829–6851. [Google Scholar] [CrossRef]
- Jiang, Q.; Lan, X.; Liu, C.; Shi, H.; Zhu, Z.; Zhao, F.; Xu, J.; Jiang, F. High-performance hybrid organic thermoelectric SWNTs/PEDOT: PSS thin-films for energy harvesting. Mater. Chem. Front. 2018, 2, 679–685. [Google Scholar] [CrossRef]
- Russ, B.; Glaudell, A.; Urban, J.J.; Chabinyc, M.L.; Segalman, R.A.; Chabinyc, R.A. Segalman, Organic thermoelectric materials for energy harvesting and temperature control. Nat. Rev. Mater. 2016, 1, 16050. [Google Scholar] [CrossRef]
- Walzer, K.; Maenning, B.; Pfeiffer, M.; Leo, K. Highly Efficient Organic Devices Based on Electrically Doped Transport Layers. Chem. Rev. 2007, 107, 1233–1271. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhang, Y.; Souri, M.; Luo, X.; Boehm, A.M.; Li, R.; Zhang, Y.; Wang, T.; Kim, D.-Y.; Mei, J.; et al. Influence of dopant size and electron affinity on the electrical conductivity and thermoelectric properties of a series of conjugated polymers. J. Mater. Chem. A 2018, 6, 16495–16505. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Roders, M.; Gumyusenge, A.; Ayzner, A.L.; Mei, J. Melt-Processing of Complementary Semiconducting Polymer Blends for High Performance Organic Transistors. Adv. Mater. 2017, 29, 1605056. [Google Scholar] [CrossRef]
- Jung, M.; Raga, S.; Ono, L.; Qi, Y. Substantial improvement of perovskite solar cells stability by pinhole-free hole transport layer with doping engineering. Sci. Rep. 2015, 5, 9863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, E.; Jeong, Y.; Oh, J.; Lee, K.; Jung, J.; Kang, Y.; Jang, J. Doping of donor-acceptor polymers with long side chains via solution mixing for advancing thermoelectric properties. Nano Energy 2019, 58, 585–595. [Google Scholar] [CrossRef]
- Zou, Y.; Huang, D.; Meng, Q.; Di, C.; Zhu, D. Correlation between Seebeck coefficient and transport energy level in poly(3-hexylthiophene). Org. Electron. 2018, 56, 125–128. [Google Scholar] [CrossRef]
- Aziz, E.; Vollmer, A.; Eisebitt, S.; Eberhardt, W.; Pingel, P.; Neher, D.; Koch, N. Localized Charge Transfer in a Molecularly Doped Conducting Polymer. Adv. Mater. 2007, 19, 3257–3260. [Google Scholar] [CrossRef]
- Alveroglu, E. Doping effect of dodecyl benzene sulphonic acid in poly(3-hexylthiophene)-P3HT-films. J. Mol. Struct. 2015, 1086, 86–92. [Google Scholar] [CrossRef]
- Gregory, S.; Menon, A.; Ye, S.; Seferos, D.; Reynolds, J.; Yee, S. Effect of Heteroatom and Doping on the Thermoelectric Properties of Poly(3-alkylchalcogenophenes). Adv. Energy Mater. 2018, 8, 1802419. [Google Scholar] [CrossRef] [Green Version]
- Thomas, E.; Davidson, E.; Katsumata, R.; Segalman, R.; Chabinyc, M. Branched Side Chains Govern Counterion Position and Doping Mechanism in Conjugated Polythiophenes. ACS Macro Lett. 2018, 7, 1492–1497. [Google Scholar] [CrossRef]
- Kroon, R.; Mengistie, D.; Kiefer, D.; Hynynen, J.; Ryan, J.; Yu, L.; Muller, C. Thermoelectric plastics: From design to synthesis, processing and structure-property relationships. Chem. Soc. Rev. 2016, 45, 6147–6164. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, J.; Ferguson, A.; Cho, C.; Grunlan, J. Carbon-nanotube-based thermoelectric materials and devices. Adv. Mater. 2018, 30, 1704386. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Yao, H.; Cui, Y.; Zou, Y.; Zhang, F.; Wang, C.; Shen, H.; Jin, W.; Zhu, J.; Diao, Y.; et al. Conjugated-Backbone Effect of Organic Small Molecules for n-Type Thermoelectric Materials with ZT over 0.2. J. Am. Chem. Soc. 2017, 139, 13013–13023. [Google Scholar] [CrossRef] [PubMed]
- Gueye, M.; Carella, A.; Massonnet, N.; Yvenou, E.; Brenet, S.; Faure-Vincent, J.; Pouget, S.; Rieutord, F.; Okuno, H.; Benayad, A.; et al. Structure and Dopant Engineering in PEDOT Thin Films: Practical Tools for a Dramatic Conductivity Enhancement. Chem. Mater. 2016, 28, 3462–3468. [Google Scholar] [CrossRef]
- Osaka, I.; McCullough, R. Advances in Molecular design and synthesis of regioregular polythiophene. Acc. Chem. Res. 2008, 41, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Feng, W.; Wang, X.; Venkatesh, R.; Ouyang, J. Significant Enhancement in the Seebeck Coefficient and Power Factor of p-Type Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) through the Incorporation of n-Type MXene. ACS Appl. Mater. Interfaces 2020, 12, 13013–13020. [Google Scholar] [CrossRef] [PubMed]
- Havinga, E.; Tenhoeve, W.; Wynberg, H. Alternate donor-acceptor small-band-gap semiconducting polymers; Polysquaraines and polycroconaines. Syntheyic Met. 1993, 55, 299–306. [Google Scholar] [CrossRef]
- Zhang, W.; Smith, J.; Watkins, S.; Gysel, R.; McGehee, M.; Salleo, A.; Kirkpatrick, J.; Ashraf, S.; Anthopoulos, T.; Heeney, M. Indacenodithiophene Semiconducting Polymers for High-Performance Air-Stable Transistors. J. Am. Chem. Soc. 2010, 132, 11437–11439. [Google Scholar] [CrossRef]
- Venkateshvaran, D.; Nikolka, M.; Sadhanala, A.; Lemaur, V.; Zelazny, M.; Kepa, M.; Hurhangee, M.; Kronemeijer, A.; Pecunia, V.; Nasrallah, I.; et al. Approaching disorder-free transport in high-mobility conjugated polymers. Nature 2014, 515, 384–388. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Zhang, Y.; Yip, H.; Sun, Y.; Davies, J.A.; Ting, C.; Chen, C.; Jen, A.K. Highly efficient indacenodithiophene-based polymeric solar cells in conventional and inverted device configurations. Org. Electron. 2011, 12, 794–801. [Google Scholar] [CrossRef]
- Wang, M.; Wang, H.; Yokoyama, T.; Liu, X.; Huang, Y.; Zhang, Y.; Nguyen, T.Q.; Aramaki, S.; Bazan, G. High open circuit voltage in regioregular narrow band gap polymer solar cells. J. Am. Chem. Soc. 2014, 136, 12576–12579. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, X.; Wang, X.; Wang, H.; Li, Y. Synthesis and Photovoltaic Properties of D-A Copolymers Based on Alkyl-Substituted Indacenodithiophene Donor Unit. Chem. Mater. 2011, 23, 4264–4270. [Google Scholar] [CrossRef]
- Wu, S.; Wu, X.; Xing, W.; Sun, Y.; Zou, Y.; Xu, W.; Zhu, D. Backbone Structure Effect on the Thermoelectric Properties of IDT-Based p-Type Conjugated Polymers. Macromol. Rapid Commun. 2019, 41, e1900322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.; Wang, L.; Pan, C.; Chen, Z.; Zhao, H.; Wang, L. Effect of backbone structure on the thermoelectric performance of indacenodithiophene-based conjugated polymers. React. Funct. Polym. 2019, 142, 1–6. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, T.; Pan, C.; Tan, G. Enhanced Thermoelectric Performance of Indacenodithiophene Benzothiadiazole Copolymer Containing Polar Side Chains and Single Wall Carbon Nanotubes Composites. Polymers 2020, 12, 848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Du, Z.; Huang, C.; Zhang, J.; Liu, S.; Fu, N.; Zhao, B.; Yang, R.; Huang, W. Angular/linear-shaped indacenodithiophene (IDT) for donor-acceptor copolymers: Geometric shape effects on physical properties and photovoltaic performance. Polymer 2019, 162, 11–19. [Google Scholar] [CrossRef]
- Xu, Y.; Chueh, C.; Yip, H.; Ding, F.; Li, Y.; Li, C.; Li, X.; Chen, W.; Jen, A. Improved charge transport and absorption coefficient in indacenodithieno[3,2-b]thiophene-based ladder-type polymer leading to highly efficient polymer solar cells. Adv. Mater. 2012, 24, 6356–6361. [Google Scholar] [CrossRef]
- Zhou, X.; Pan, C.; Gao, C.; Shinohara, A.; Yin, X.; Wang, L.; Li, Y.; Jiang, Q.; Yang, C.; Wang, L. Thermelectrics of two-dimensional conjugated benzodithiophene-based polymers: Density-of-states enhancement and semi-metallic behavior. J. Mater. Chem. A 2019, 7, 10422–10430. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef]
- Gayner, C.; Kar, K. Recent advances in thermoelectric materials. Prog. Mater. Sci. 2016, 83, 330–382. [Google Scholar] [CrossRef]
- Yamamoto, J.; Furukawa, Y. Electronic and vibrational spectra of positive polarons and bipolarons in regioregular poly(3-hexylthiophene) doped with ferric chloride. J. Phys. Chem. B 2015, 119, 4788–4794. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ouyang, J.; Tang, A.; Xiao, Y.; Li, X.; Dong, X.; Yu, Y. Electrochemical synthesis and photocatalytic property of cuprous oxide nanoparticles. Mater. Res. Bull. 2006, 41, 1310–1318. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, F.; Di, C.; Yan, T.; Zou, Y.; Zhou, X.; Zhu, D.; Wang, J.; Pei, J. Toward High Performance n-Type Thermoelectric Materials by Rational Modification of BDPPV Backbones. J. Am. Chem. Soc. 2015, 137, 6979–6982. [Google Scholar] [CrossRef] [PubMed]
- Scholes, D.; Yee, P.; Lindemuth, J.; Kang, H.; Onorato, J.; Ghosh, R.; Luscombe, C.; Spano, F.; Tolbert, S.; Schwartz, B. The Effects of Crystallinity on Charge Transport and the Structure of Sequentially Processed F4TCNQ-Doped Conjugated Polymer Films. Adv. Funct. Mater. 2017, 27, 1702654. [Google Scholar] [CrossRef]
- Xing, K.; Fahlman, M.; Chen, X.; Inganas, O.; Salaneck, W. The electronic structure of poly(3,4-ethylene-dioxythiophene): Studied by XPS and UPS. Synthetic Met. 1997, 89, 161–165. [Google Scholar] [CrossRef]
- Liang, Z.; Boland, M.; Butrouna, K.; Strachan, D.; Graham, K. Increased power factors of organic–inorganic nanocomposite thermoelectric materials and the role of energy filtering. J. Mater. Chem. A 2017, 5, 15891–15900. [Google Scholar] [CrossRef]
- Mendez, H.; Heimel, G.; Opitz, A.; Sauer, K.; Barkowski, P.; Oehzelt, M.; Soeda, J.; Okamoto, T.; Takeya, J.; Arlin, J.; et al. Doping of organic semiconductors: Impact of dopant strength and electronic coupling. Angew. Chem. 2013, 52, 7751–7755. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Glaudell, A.; Peterson, K.; Thomas, E.; O’Hara, K.; Lim, E.; Chabinyc, M. Morphology controls the thermoelectric power factor of doped semiconducting polymer. Sci. Adv. 2017, 3, 1700434. [Google Scholar] [CrossRef] [Green Version]







| Polymer | EHOMO (eV) | ELUMO (eV) | Egec (eV) | Egopt (eV) | λonset (nm) | EgDFT (eV) | Carrier Mobility (10−5 cm2 v−1 s−1) |
|---|---|---|---|---|---|---|---|
| P1 | −5.77 | −3.21 | 2.56 | 2.02 | 613 | 2.50 | 3.48 |
| P2 | −5.78 | −3.28 | 2.50 | 2.00 | 617 | 2.40 | 13.35 |
| P3 | −5.77 | −3.49 | 2.28 | 1.70 | 730 | 2.10 | 354 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Xie, D.; Xu, J.; Pan, C. Structure and Doping Optimization of IDT-Based Copolymers for Thermoelectrics. Polymers 2020, 12, 1463. https://doi.org/10.3390/polym12071463
Liu T, Xie D, Xu J, Pan C. Structure and Doping Optimization of IDT-Based Copolymers for Thermoelectrics. Polymers. 2020; 12(7):1463. https://doi.org/10.3390/polym12071463
Chicago/Turabian StyleLiu, Tongchao, Dexun Xie, Jinjia Xu, and Chengjun Pan. 2020. "Structure and Doping Optimization of IDT-Based Copolymers for Thermoelectrics" Polymers 12, no. 7: 1463. https://doi.org/10.3390/polym12071463
APA StyleLiu, T., Xie, D., Xu, J., & Pan, C. (2020). Structure and Doping Optimization of IDT-Based Copolymers for Thermoelectrics. Polymers, 12(7), 1463. https://doi.org/10.3390/polym12071463