Bio-Based Polyamide 1010 with a Halogen-Free Flame Retardant Based on Melamine–Gallic Acid Complex
Abstract
:1. Introduction
- -
- the hydrolysis of the amide bond below the decomposition temperature (Td);
- -
- homolytic scission of the C–C, C–H, C–N bonds at temperatures above Td or simultaneously with hydrolysis;
- -
- cyclization and homolytic scission of the products resultingfrom both reactions;
- -
- side reactions that produce carbon oxide, nitriles, ammonia, hydrocarbons.
2. Materials and Methods
2.1. Materials
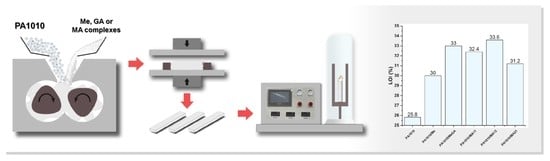
2.2. Preparation of Complexes and Composites
2.3. Characterization
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. Thermal Characterization
2.3.3. Dynamic Mechanical Analysis (DMA)
2.3.4. X-ray Diffraction Analysis (XRD)
2.3.5. Flame Retardant Properties
3. Results and Discussion
3.1. Characterization of Me, GA, and MA Complexes
3.1.1. FTIR Analysis
3.1.2. Thermogravimetric Analysis
3.2. Characterization of PA1010 Composites
3.2.1. FTIR Analysis
3.2.2. Thermogravimetric Analysis
3.2.3. Differential Scanning Analysis
3.2.4. Dynamic Mechanical Analysis
3.2.5. X-ray Diffraction Analysis
3.2.6. Flame Retardant Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kyulavska, M.; Toncheva-Moncheva, N.; Rydz, J. Biobased polyamide ecomaterials and their susceptibility to biodegradation. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–34. [Google Scholar]
- Rusu, D.; Boyer, S.; Lacrampe, M.F.; Krawczak, P. Bioplastics in automotive applications. Handb. Bioplast Biocompos. Eng. Appl. 2012, 81, 397. [Google Scholar]
- Moran, C.S.; Barthelon, A.; Pearsall, A.; Mittal, V.; Dorgan, J.R. Biorenewable blends of polyamide-4,10 and polyamide-6,10. J. Appl. Polym. Sci. 2016, 133, 43626. [Google Scholar] [CrossRef]
- Kind, S.; Neubauer, S.; Becker, J.; Yamamoto, M.; Völkert, M.; Abendroth, G.V.; Zelder, O.; Wittmann, C. From zero to hero–Production of bio-based nylon from renewable resources using engineered Corynebacteriumglutamicum. Metab. Eng. 2014, 25, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Ogunsona, E.O.; Misra, M.; Mohanty, A.K. Sustainable biocomposites from biobased polyamide 6,10 and biocarbon from pyrolyzedmiscanthusfibers. J. Appl. Polym. Sci. 2017, 134, 44221. [Google Scholar] [CrossRef]
- Kuciel, S.; Romańska, P.; Jakubowska, P. Properties of composites based on polyamide 10.10 reinforced with carbon fibers. Polimery 2016, 61, 106–112. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Frone, A.N.; Nicolae, C. Micro- and nano-mechanical characterization of polyamide 11 and its composites containing cellulose nanofibers. Eur. Polym. J. 2013, 49, 3857–3866. [Google Scholar] [CrossRef]
- Pagacz, J.; Raftopoulos, K.N.; Leszczyńska, A.; Pielichowski, K. Bio-polyamides based on renewable raw materials. J. Therm. Anal. Calorim. 2016, 123, 1225–1237. [Google Scholar] [CrossRef] [Green Version]
- Quiles-Carrillo, L.; Montanes, N.; Boronat, T.; Balart, R.; Torres-Giner, S. Evaluation of the engineering performance of different bio-based aliphatic homopolyamide tubes prepared by profile extrusion. Polym. Test. 2017, 61, 421–429. [Google Scholar] [CrossRef]
- Matzen, M.; Kandola, B.; Huth, C.; Schartel, B. Influence of Flame retardants on the melt dripping behaviour of thermoplastic polymers. Materials 2015, 8, 5621–5646. [Google Scholar] [CrossRef] [Green Version]
- Herrera, M.; Matuschek, G.; Kettrup, A. Main products and kinetics of the thermal degradation of polyamides. Chemosphere 2001, 42, 601–607. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. Thermal degradation of nylon polymers. Polym. Int. 2000, 49, 943–948. [Google Scholar] [CrossRef]
- Kausar, A. Polyamide 1010/Polythioamide Blend Reinforced with Graphene Nanoplatelet for Automotive Part Application. Adv. Mater. Sci. 2017, 17, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Battegazzore, D.; Alongi, J.; Fontaine, G.; Frache, A.; Bourbigot, S.; Malucelli, G. Bulk vs. surface flame retardancy of fully bio-based polyamide 10,10. RSC Adv. 2015, 5, 39424–39432. [Google Scholar] [CrossRef]
- Levinṭa, N.; Vuluga, Z.; Teodorescu, M.; Corobea, M.C. Halogen-free flame retardants for application in thermoplastics based on condensation polymers. SN Appl. Sci. 2019, 1, 422. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Z.; Xu, M.; Li, B. Synergistic effects of sepiolite on the flame retardant properties and thermal degradation behaviors of polyamide 66/aluminumdiethylphosphinate composites. Polym. Degrad. Stab. 2015, 117, 66–74. [Google Scholar] [CrossRef]
- Zhan, Z.; Li, B.; Xu, M.; Guo, Z. Synergistic effects of nano-silica on aluminumdiethylphosphinate/polyamide 66 system for fire retardancy. High Perform. Polym. 2016, 28, 140–146. [Google Scholar] [CrossRef]
- Li, L.; Chen, G.; Liu, W.; Li, J.; Zhang, S. The anti-dripping intumescent flame retardant finishing for nylon-6,6 fabric. Polym. Degrad. Stab. 2009, 94, 996–1000. [Google Scholar] [CrossRef]
- Sun, J.; Gu, X.; Coquelle, M.; Bourbigot, S.; Duquesne, S.; Casetta, M.; Zhang, S. Effects of melamine polyphosphate and halloysite nanotubes on the flammability and thermal behavior of polyamide 6. Polym. Adv. Technol. 2014, 25, 1552–1559. [Google Scholar] [CrossRef]
- Luo, D.; Duan, W.; Liu, Y.; Chen, N.; Wang, Q. Melamine cyanurate surface treated by nylon of low molecular weight to prepare flame-retardant polyamide 66 with high flowability. Fire Mater. 2019, 43, 323–331. [Google Scholar] [CrossRef]
- Lu, X.; Qiao, X.; Yang, T.; Sun, K.; Chen, X. Preparation and properties of environmental friendly nonhalogen flame retardant melamine cyanurate/nylon 66 composites. J. Appl. Polym. Sci. 2011, 122, 1688–1697. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q. The investigation on the flame retardancy mechanism of nitrogen flame retardant melamine cyanurate in polyamide 6. J. Polym. Res. 2009, 16, 583–589. [Google Scholar] [CrossRef]
- Jin, X.; Chen, C.; Sun, J.; Zhang, X.; Gu, X.; Zhang, S. The synergism between melamine and expandable graphite on improving the flame retardancy of polyamide 11. High Perform. Polym. 2017, 29, 77–86. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Li, M.; Du, M.; Li, X.; Li, Y. A Review of a Class of Emerging Contaminants: The Classification, Distribution, Intensity of Consumption, Synthesis Routes, Environmental Effects and Expectation of Pollution Abatement to Organophosphate Flame Retardants (OPFRs). Int. J. Mol. Sci. 2019, 20, 2874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, Y.; Zhao, Y.; Yan, N. Synthesis and Characterization of Biobased Melamine Formaldehyde Resins from Bark Extractives. Ind. Eng. Chem. Res. 2014, 53, 11228–11238. [Google Scholar] [CrossRef]
- Isbasar, C.; Hacaloglu, J. Investigation of thermal degradation characteristics of polyamide-6 containing melamine or melamine cyanurate via direct pyrolysis mass spectrometry. J. Anal. Appl. Pyrolysis 2012, 98, 221–230. [Google Scholar] [CrossRef]
- Lotsch, B.V.; Schnick, W. New light on an old story: Formation of melam during thermal condensation of melamine. Chem. Eur. J. 2007, 13, 4956–4968. [Google Scholar] [CrossRef]
- Levchik, S.V.; Costa, L.; Camino, G. Effect of ammonium polyphosphate on combustion and thermal degradation of aliphatic polyamides. Makromol. Chem. Macromol. Symp. 1993, 74, 95–99. [Google Scholar] [CrossRef]
- Karamać, M.; Kosinska, A.; Pegg, R.B. Content of Gallic acid in selected plant extracts. Pol. J. Food Nutr. Sci. 2006, 15, 55–58. [Google Scholar]
- Gnawali, G.R.; Acharya, P.; Rajbhandari, M. Isolation of Gallic Acid and Estimation of Total Phenolic Content in Some Medicinal Plants and Their Antioxidant Activity. Nepal J. Sci. Technol. 2013, 14, 95–102. [Google Scholar]
- Wang, X.-H.; Cai, C.; Li, X.-M. Optimal extraction of gallic acid from suaedaglaucabge. leaves and enhanced efficiency by ionic liquids. Int. J. Chem. Eng. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Usman, M.; Hussein, M.; Fakurazi, S.; Masarudin, J.; AhmasSaad, F.F. Gadolinium-Doped gallic acid-zinc/aluminium-layered double hydroxide/gold theranostic nanoparticles for a bimodal magnetic resonance imaging and drug delivery system. Nanomaterials 2017, 7, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, M.A.; Collins, P.B. Handbook on Gallic Acid: Natural Occurrences, Antioxidant Properties And Health Implications; Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 1–350. [Google Scholar]
- Saha, A.; Roy, B.; Garai, A.; Nandi, A.K. Two-Component thermoreversible hydrogels of melamine and gallic acid. Langmuir 2009, 25, 8457–8461. [Google Scholar] [CrossRef] [PubMed]
- Farrokhnia, M.; Karimi, S.; Askarian, S. Strong hydrogen bonding of gallic acid during synthesis of an efficient agnps colorimetric sensor for melamine detection via dis-synthesis strategy. ACS Sustain. Chem. Eng. 2019, 7, 6672–6684. [Google Scholar] [CrossRef]
- Su, Y.-L.; Cheng, S.-H. Sensitive and selective determination of gallic acid in green tea samples based on an electrochemical platform of poly(melamine) film. Anal. Chim. Acta 2015, 901, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Howell, B.; Oberdorfer, K.; Ostrander, E. Phosphorus flame retardants for polymeric materials from gallic acid and other naturally occurring multihydroxybenzoic acids. Int. J. Polym. Sci. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Karaseva, V.; Bergeret, A.; Lacoste, C.; Fulcrand, H.; Ferry, L. New biosourced flame retardant agents based on gallic and ellagic acids for epoxy resins. Molecules 2019, 24, 4305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M.; Mestry, S.; Khuntia, S.P.; Mhaske, S. Gallic acid-derived phosphorus-based flame-retardant multifunctional crosslinking agent for PU coating. J. Coat. Technol. Res. 2020, 17, 293–303. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Boronat, T.; Montanes, N.; Balart, R.; Torres-Giner, S. Injection-molded parts of fully bio-based polyamide 1010 strengthened with waste derived slate fiberspretreated with glycidyl- and amino-silane coupling agents. Polym. Test. 2019, 77, 105875. [Google Scholar] [CrossRef]
- ISO (The International Organization for Standardization). ISO4589-2:2017, Plastics—Determination of Burning Behaviour by Oxygen Index—Part 2: Ambient-Temperature Test; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Sangeetha, V.; Kanagathara, N.; Sumathi, R.; Sivakumar, N.; Anbalagan, G. Spectral and thermal degradation of melamine cyanurate. J. Mater. 2013, 2013, 262094. [Google Scholar] [CrossRef] [Green Version]
- Alberti, A.; Granato, D.; Nogueira, A.; Mafra, L.I.; Colman, T.A.D.; Schnitzler, E. Modelling the thermal decomposition of 3,4,5-trihydroxybenzoic acid using ordinary least square regression. Int. Food Res. J. 2016, 23, 30–33. [Google Scholar]
- Murthy, N.S. Hydrogen bonding, mobility, and structural transitions in aliphatic polyamides. J. Polym. Sci. B Polym. Phys. 2006, 44, 1763–1782. [Google Scholar] [CrossRef]
- Vasanthan, N.; Salem, D.R. Infrared spectroscopic characterization of oriented polyamide 66: Band assignment and crystallinity measurement. J. Polym. Sci. B Polym. Phys. 2000, 38, 516–524. [Google Scholar] [CrossRef]
- Tashiro, K.; Yamamoto, H. Structural evolution mechanism of crystalline polymers in the isothermal melt-crystallization process: A proposition based on simultaneous WAXD/SAXS/FTIR measurements. Polymers 2019, 11, 1316. [Google Scholar] [CrossRef] [Green Version]
- Ishisue, T.; Okamoto, M.; Tashiro, K. Real-time investigation of crystallization in nylon 6-clay nano-composite probed by infrared spectroscopy. Polymer 2010, 51, 5585–5591. [Google Scholar] [CrossRef]
- Vasiljević, J.; Čolović, M.; Jerman, I.; Simončič, B.; Demšar, A.; Samaki, Y.; Šobak, M.; Šest, E.; Golja, B.; Leskovšek, M.; et al. In situ prepared polyamide 6/DOPO-derivative nanocomposite for melt-spinning of flame retardant textile filaments. Polym. Degrad. Stab. 2019, 166, 50–59. [Google Scholar] [CrossRef]
- Vasiljević, J.Č.; Čolović, M.; ČelanKorošin, N.; Šobak, M.; Štirn, Ž.; Jerman, I. Effect of different flame-retardant bridged dopo derivatives on properties of in situ produced fiber-forming polyamide 6. Polymers 2020, 12, 657. [Google Scholar]
- Wentao, L.; Liu, H.; Sun, A.; Yoo, Y.; He, S.; Zhu, C.; Yang, M. The thermal behavior of γ-pa1010: Evolution of structure and morphology in the simultaneous thermal stretched films. Materials 2020, 13, 1722. [Google Scholar]
- Zhishen, M.; Qingbo, M.; Jinhua, F.; Hongfang, Z.; Donglin, C. Crystal structure and thermodynamic parameters of Nylon-1010. Polym Int. 1993, 32, 53–60. [Google Scholar] [CrossRef]
- Criado, P.; Fraschini, C.; Salmieri, S.; Becher, D.; Safrany, A.; Lacroix, M. Free radical grafting of gallic acid (GA) on cellulose nanocrystals (CNCS) and evaluation of antioxidant reinforced gellan gum films. Radiat. Phys. Chem. 2016, 118, 61–69. [Google Scholar] [CrossRef]










| Samples | PA1010 (wt %) | Me (wt %) | GA (wt %) | MA11 (wt %) | MA12 (wt %) | MA21 (wt %) |
|---|---|---|---|---|---|---|
| PA1010 | 100 | - | - | - | - | - |
| PA1010/Me | 90 | 10 | - | - | - | - |
| PA1010/MeGA | 90 | 5 | 5 | - | - | - |
| PA1010/MA11 | 90 | - | - | 10 | - | - |
| PA1010/MA12 | 90 | - | - | - | 10 | - |
| PA1010/MA21 | 90 | - | - | - | - | 10 |
| Sample | Ton | Tmax | Residue at Tmax | Residue at 600 °C |
|---|---|---|---|---|
| (°C) | °C | % | % | |
| Me | 323.1 | 335.5 | 24.37 | 0.16 |
| GA | 58.5 196.7 318.1 | 78.1 264.9 339.3 | 96.89 76.17 44.51 | 11.23 |
| MA11 | 64.2 216.0 252.8 272.0 | 79.4 222.8 260.7 280.8 | 91.59 82.94 35.00 10.30 | 0.5 |
| MA12 | 78.9 256.9 274.0 | 92.9 262.1 295.4 | 90.1 34.2 8.8 | 0.4 |
| MA21 | 30.8 220.6 253.8 266.0 | 42.7 223.9 260.0 279.0 | 97.6 87.4 36.8 9.8 | 0.4 |
| PA1010 | PA1010/MA12 | Band Assignment | |
|---|---|---|---|
| 3303 | 3303 | N–H stretching vibration | General |
| 3075 | 3075 | N–H stretch and amide II overtone | General |
| 2919 | 2918 | CH2 asymmetric stretching | General |
| 2850 | 2851 | CH2 symmetric stretching | General |
| 1738 | 1738 | C=O stretch (ester) | General |
| 1632 | 1633 | Amide I band (C=O stretching) | General |
| 1538 | 1539 | Amide II band (N–H in-plane bending coupled with C–N and C–O stretch) | General |
| 1470 | 1467 | CH2 scissoring not adjacent to the amide group | α-structure |
| 1418 | 1420 | CH2 scissoring | α-structure |
| 1435 | 1435 | CH2 scissors vibration | γ-structure |
| 1359 | 1359 | CH2 twist-wagging | γ-structure |
| 1237 | 1236 | CH2 twist-wagging | γ-structure |
| 1190 | 1189 | CH2 twist-wagging | α-structure |
| 940 | 941 | Vibration of the N-vicinal CH2 group coupled amide III “crystalline band” | α-structure |
| 720 | 718 | Rocking mode of CH2 | α-structure γ-structure |
| Sample | Nitrogen | Air | ||||||
|---|---|---|---|---|---|---|---|---|
| Ton | Tmax | Residue at Tmax | Residue at 600 °C | Ton | Tmax | Residue at Tmax | Residue at 600 °C | |
| (°C) | (°C) | (%) | (%) | (°C) | (°C) | (%) | (%) | |
| PA1010 | 451.4 | 466.1 | 36.56 | 0.16 | 421.8 446.7 | 426.7 451.8 | 80.66 50.42 | 0.38 |
| PA1010/Me | 272.4 457.1 | 324.1 467.9 | 92.63 31.16 | 0.19 | 417.1 452.1 | 419.9 459.4 | 78.90 38.94 | 0.29 |
| PA1010/MeGA | 231.4 455.8 | 273.7 468.6 | 92.72 31.32 | 0.51 | 450.7 494.4 | 461.7 522.5 | 41.16 5.964 | 0.42 |
| PA1010/MA11 | 223.1 459.1 | 261.5 468.6 | 94.98 32.17 | 0.38 | 450.1 489.1 | 462.3 497.5 | 39.15 7.79 | 0.58 |
| PA1010/MA12 | 232.7 464.5 | 257.6 469.8 | 94.89 32.65 | 0.40 | 454.8 488.4 | 462.3 496.2 | 40.86 6.42 | 0.54 |
| PA1010/MA21 | 230.0 466.7 | 270.5 470.5 | 94.17 32.00 | 0.60 | 449.6 489.6 | 460.7 499.7 | 44.65 6.10 | 0.25 |
| Sample | Tg | Tm1/Tm2 | ΔHm | ΔHm1/Hm2 | Tc | ΔHc |
|---|---|---|---|---|---|---|
| °C | °C | J/g | J/g | °C | J/g | |
| PA1010 | 36.6 | 189.3/198.2 | 75.94 | 47.25/28.69 | 178.9 | 52.94 |
| PA1010/Me | 37.5 | 188.5/198.6 | 66.26 | 38.82/27.44 | 179.4 | 46.85 |
| PA1010/MeGA | 23.9 | 184.0/194.4 | 65.74 | 36.65/29.09 | 175.9 | 46.63 |
| PA1010/MA11 | 24.0 | 183.2/195.4 | 68.21 | 37.71/30.51 | 176.0 | 43.44 |
| PA1010/MA12 | 25.9 | 184.8/195.1 | 63.18 | 35.06/28.12 | 176.7 | 40.50 |
| PA1010/MA21 | 18.7 | 180.9/192.0 | 68.46 | 35.05/33.42 | 173.9 | 41.76 |
| Samples | Positions (degree) | Full Width at the Half Maximum (FWHM) | Spacing/nm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PA1010 | 8.37 | 20.06 | 23.7 | 1.57 | 1.65 | 2.08 | 1.058 | 0.449 | 0.383 |
| PA1010/MeGA | 8.44 | 20.21 | 23.8 | 2.38 | 1.15 | 2.42 | 1.049 | 0.446 | 0.382 |
| PA1010/MA11 | 8.50 | 20.32 | 24.08 | 1.81 | 1.19 | 2.30 | 1.042 | 0.444 | 0.377 |
| PA1010/MA12 | 8.15 | 20.34 | 24.04 | 2.74 | 1.45 | 1.95 | 1.088 | 0.443 | 0.378 |
| PA1010/MA21 | 8.50 | 20.28 | 24.12 | 2.36 | 1.11 | 2.25 | 1.042 | 0.444 | 0.377 |
| Sample | t1 | t2 | Cotton Indicator Ignition | Classification |
|---|---|---|---|---|
| (s) | (s) | |||
| PA1010 | 25.0 ± 1 | 20.0 ± 1 | Yes | V-2 |
| PA1010/Me | 0 | 0 | No | V-0 |
| PA1010/MeGA | 0 | 0 | No | V-0 |
| PA1010/MA11 | 0 | 0 | No | V-0 |
| PA1010/MA12 | 0 | 0 | No | V-0 |
| PA1010/MA21 | 0 | 0 | No | V-0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levinta, N.; Corobea, M.C.; Vuluga, Z.; Nicolae, C.-A.; Gabor, A.R.; Raditoiu, V.; Osiac, M.; Teodorescu, G.-M.; Teodorescu, M. Bio-Based Polyamide 1010 with a Halogen-Free Flame Retardant Based on Melamine–Gallic Acid Complex. Polymers 2020, 12, 1482. https://doi.org/10.3390/polym12071482
Levinta N, Corobea MC, Vuluga Z, Nicolae C-A, Gabor AR, Raditoiu V, Osiac M, Teodorescu G-M, Teodorescu M. Bio-Based Polyamide 1010 with a Halogen-Free Flame Retardant Based on Melamine–Gallic Acid Complex. Polymers. 2020; 12(7):1482. https://doi.org/10.3390/polym12071482
Chicago/Turabian StyleLevinta, Nicoleta, Mihai Cosmin Corobea, Zina Vuluga, Cristian-Andi Nicolae, Augusta Raluca Gabor, Valentin Raditoiu, Mariana Osiac, George-Mihail Teodorescu, and Mircea Teodorescu. 2020. "Bio-Based Polyamide 1010 with a Halogen-Free Flame Retardant Based on Melamine–Gallic Acid Complex" Polymers 12, no. 7: 1482. https://doi.org/10.3390/polym12071482
APA StyleLevinta, N., Corobea, M. C., Vuluga, Z., Nicolae, C. -A., Gabor, A. R., Raditoiu, V., Osiac, M., Teodorescu, G. -M., & Teodorescu, M. (2020). Bio-Based Polyamide 1010 with a Halogen-Free Flame Retardant Based on Melamine–Gallic Acid Complex. Polymers, 12(7), 1482. https://doi.org/10.3390/polym12071482