Nanoformulation Design Including MamC-Mediated Biomimetic Nanoparticles Allows the Simultaneous Application of Targeted Drug Delivery and Magnetic Hyperthermia
Abstract
:1. Introduction
2. Materials and Methods
2.1. M and BM Synthesis
2.2. Functionalization of the BM
2.3. Nanoparticles Characterization
2.4. Stability Evaluation
2.5. Hyperthermia Experiments
2.6. Nanoformulation as Nanocarriers: Effect of Hyperthermia on DOXO Release
2.7. Cell Cultures
2.8. Cytocompatibility and Cytotoxicity of the Nanoassemblies
2.9. Statistical Analysis
3. Results and Discussion
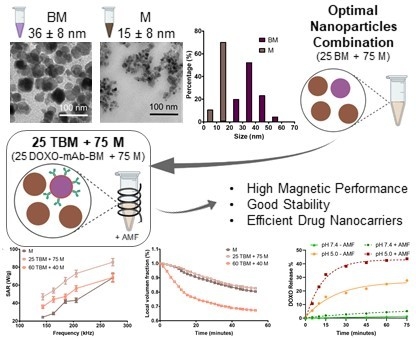
3.1. Morphology and Particle Size
3.2. Magnetic Hyperthermia Responses
3.3. Colloidal Stability
3.4. Nanoformulation as DOXO Nanocarrier. Synergy of Magnetic Hyperthermia and of pH Decrease in the Drug Release
3.5. Cytotoxicity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yagawa, Y.; Tanigawa, K.; Kobayashi, Y.; Yamamoto, M. Cancer immunity and therapy using hyperthermia with immunotherapy, radiotherapy, chemotherapy, and surgery. J. Cancer Metastasis Treat. 2017, 3, 218. [Google Scholar] [CrossRef]
- Phung, D.C.; Nguyen, H.T.; Phuong Tran, T.T.; Jin, S.G.; Yong, C.S.; Truong, D.H.; Tran, T.H.; Kim, J.O. Combined hyperthermia and chemotherapy as a synergistic anticancer treatment. J. Pharm. Investig. 2019, 49, 519–526. [Google Scholar] [CrossRef]
- Garanina, A.S.; Naumenko, V.A.; Nikitin, A.A.; Myrovali, E.; Petukhova, A.Y.; Klimyuk, S.V.; Nalench, Y.A.; Ilyasov, A.R.; Vodopyanov, S.S.; Erofeev, A.S.; et al. Temperature-controlled magnetic nanoparticles hyperthermia inhibits primary tumor growth and metastases dissemination. Nanomed. Nanotechnol. Biol. Med. 2020, 25, 102171. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef]
- Kang, J.K.; Kim, J.C.; Shin, Y.; Han, S.M.; Won, W.R.; Her, J.; Park, J.Y.; Oh, K.T. Principles and applications of nanomaterial-based hyperthermia in cancer therapy. Arch. Pharm. Res. 2020, 43, 46–57. [Google Scholar] [CrossRef]
- Häring, M.; Schiller, J.; Mayr, J.; Grijalvo, S.; Eritja, R.; Díaz, D. Magnetic Gel Composites for Hyperthermia Cancer Therapy. Gels 2015, 1, 135–161. [Google Scholar] [CrossRef] [Green Version]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Iglesias, G.R.; Reyes-Ortega, F.; Fernandez, B.L.C.; Delgado, Á.V. Hyperthermia-triggered gemcitabine release from polymer-coated magnetite nanoparticles. Polymers 2018, 10, 269. [Google Scholar] [CrossRef] [Green Version]
- Husain, Q. Magnetic nanoparticles as a tool for the immobilization/stabilization of hydrolases and their applications: An overview. Appl. Chem. 2016, 6, 1585–1606. [Google Scholar]
- Mehdaoui, B.; Meffre, A.; Carrey, J.; Lachaize, S.; Lacroix, L.-M.; Gougeon, M.; Chaudret, B.; Respaud, M. Optimal Size of Nanoparticles for Magnetic Hyperthermia: A Combined Theoretical and Experimental Study. Adv. Funct. Mater. 2011, 21, 4573–4581. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, J.; Zeng, F.; Elkins, K.; Xing, M.; Ghimire, M.; Yoon, S.; Mishra, S.R.; Liu, J.P. Size-dependent magnetic and inductive heating properties of Fe3O4 nanoparticles: Scaling laws across the superparamagnetic size. Phys. Chem. Chem. Phys. 2018, 20, 12879–12887. [Google Scholar] [CrossRef]
- Briceno, S.; Silva, P.; Bramer-Escamilla, W.; Zabala, J.; Alcala, O.; Guari, Y.; Larionova, J.; Long, J.; Briceño, S.; Long, J. Magnetic water-soluble rhamnose-coated Mn1-xCoxFe2O4 nanoparticles as potential heating agents for hyperthermia. Biointerface Res. Appl. Chem. 2015, 5, 910–915. [Google Scholar]
- Yüksel, Y. Effects of the particle size and shape of the magnetic nanoparticles on the magnetic hyperthermia and exchange bias properties. Phys. B Condens. Matter 2019, 575, 411689. [Google Scholar] [CrossRef]
- Jabalera, Y.; Fernández-Vivas, A.; Iglesias, G.R.; Delgado, Á.V.; Jimenez-Lopez, C. Magnetoliposomes of mixed biomimetic and inorganic magnetic nanoparticles as enhanced hyperthermia agents. Colloids Surf. B Biointerfaces 2019, 183, 110435. [Google Scholar] [CrossRef]
- Li, Z.; Kawashita, M.; Araki, N.; Mitsumori, M.; Hiraoka, M.; Doi, M. Magnetite nanoparticles with high heating efficiencies for application in the hyperthermia of cancer. Mater. Sci. Eng. C 2010, 30, 990–996. [Google Scholar] [CrossRef]
- Li, Q.; Kartikowati, C.W.; Horie, S.; Ogi, T.; Iwaki, T.; Okuyama, K. Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 9894. [Google Scholar] [CrossRef]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Preparation, conjugation and delivery. Nanomedicine 2018, 13, 929–952. [Google Scholar] [CrossRef]
- Valverde-Tercedor, C.; Montalbán-López, M.; Perez-Gonzalez, T.; Sanchez-Quesada, M.S.; Prozorov, T.; Pineda-Molina, E.; Fernandez-Vivas, M.A.; Rodriguez-Navarro, A.B.; Trubitsyn, D.; Bazylinski, D.A.; et al. Size control of in vitro synthesized magnetite crystals by the MamC protein of Magnetococcus marinus strain MC-1. Appl. Microbiol. Biotechnol. 2015, 99, 5109–5121. [Google Scholar] [CrossRef]
- García Rubia, G.; Peigneux, A.; Jabalera, Y.; Puerma, J.; Oltolina, F.; Elert, K.; Colangelo, D.; Gómez Morales, J.; Prat, M.; Jimenez-Lopez, C. PH-Dependent Adsorption Release of Doxorubicin on MamC-Biomimetic Magnetite Nanoparticles. Langmuir 2018, 34, 13713–13724. [Google Scholar] [CrossRef]
- Jabalera, Y.; Garcia-Pinel, B.; Ortiz, R.; Iglesias, G.; Cabeza, L.; Prados, J.; Jimenez-Lopez, C.; Melguizo, C. Oxaliplatin–Biomimetic Magnetic Nanoparticle Assemblies for Colon Cancer-Targeted Chemotherapy: An In Vitro Study. Pharmaceutics 2019, 11, 395. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, G.R.; Jabalera, Y.; Peigneux, A.; Checa Fernández, B.L.; Delgado, Á.V.; Jimenez-Lopez, C.; Iglesias, G.R.; Jabalera, Y.; Peigneux, A.; Checa Fernández, B.L.; et al. Enhancement of Magnetic Hyperthermia by Mixing Synthetic Inorganic and Biomimetic Magnetic Nanoparticles. Pharmaceutics 2019, 11, 273. [Google Scholar] [CrossRef] [Green Version]
- Perez-Gonzalez, T.; Jimenez-Lopez, C.; Neal, A.L.; Rull-Perez, F.; Rodriguez-Navarro, A.; Fernandez-Vivas, A.; Iañez-Pareja, E. Magnetite biomineralization induced by Shewanella oneidensis. Geochim. Cosmochim. Acta 2010, 74, 967–979. [Google Scholar] [CrossRef]
- Prat, M.; Morra, I.; Bussolati, G.; Comoglio, P.M. CAR-3, a monoclonal antibody-defined antigen expressed on human carcinomas. Cancer Res. 1985, 45, 5799–5807. [Google Scholar]
- Prat, M.; Medico, E.; Rossino, P.; Garrino, C.; Comoglio, P.M. Biochemical and immunological properties of the human carcinoma-associated CAR-3 epitope defined by the monoclonal antibody AR-3. Cancer Res. 1989, 49, 1415–1421. [Google Scholar]
- Iafisco, M.; Delgado-Lopez, J.M.; Varoni, E.M.; Tampieri, A.; Rimondini, L.; Gomez-Morales, J.; Prat, M. Cell Surface Receptor Targeted Biomimetic Apatite Nanocrystals for Cancer Therapy. Small 2013, 9, 3834–3844. [Google Scholar] [CrossRef]
- Obaidat, I.; Issa, B.; Haik, Y. Magnetic Properties of Magnetic Nanoparticles for Efficient Hyperthermia. Nanomaterials 2015, 5, 63–89. [Google Scholar] [CrossRef] [Green Version]
- Wildeboer, R.R.; Southern, P.; Pankhurst, Q.A. On the reliable measurement of specific absorption rates and intrinsic loss parameters in magnetic hyperthermia materials. J. Phys. D. Appl. Phys. 2014, 47, 495003. [Google Scholar] [CrossRef]
- Peigneux, A.; Oltolina, F.; Colangelo, D.; Iglesias, G.R.; Delgado, A.V.; Prat, M.; Jimenez-Lopez, C. Functionalized Biomimetic Magnetic Nanoparticles as Effective Nanocarriers for Targeted Chemotherapy. Part. Part. Syst. Charact. 2019, 36, 1900057. [Google Scholar] [CrossRef]
- Jabalera, Y.; Sola-Leyva, A.; Peigneux, A.; Vurro, F.; Iglesias, G.R.; Vilchez-Garcia, J.; Pérez-Prieto, I.; Aguilar-Troyano, F.J.; López-Cara, L.C.; Carrasco-Jiménez, M.P.; et al. Biomimetic magnetic nanocarriers drive choline kinase alpha inhibitor inside cancer cells for combined chemo-hyperthermia therapy. Pharmaceutics 2019, 11, 408. [Google Scholar] [CrossRef] [Green Version]
- Oltolina, F.; Gregoletto, L.; Colangelo, D.; Gómez-Morales, J.; Delgado-López, J.M.; Prat, M. Monoclonal antibody-targeted fluorescein-5-isothiocyanate-labeled biomimetic nanoapatites: A promising fluorescent probe for imaging applications. Langmuir 2015, 31, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, U.; Bukhari, N.I.; Rana, N.F.; Rehman, M.; Hussain, K.; Abbas, N.; Mehmood, A.; Raza, A. Doxorubicin-loaded quaternary ammonium palmitoyl glycol chitosan polymeric nanoformulation: Uptake by cells and organs. Int. J. Nanomed. 2019, 14, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Tse, W.H.; Zhang, J. Deposition of Antibody Modified Upconversion Nanoparticles on Glass by a Laser-Assisted Method to Improve the Performance of Cell Culture. Nanoscale Res. Lett. 2019, 14, 101. [Google Scholar] [CrossRef]
- Glassford, S.E.; Byrne, B.; Kazarian, S.G. Recent applications of ATR FTIR spectroscopy and imaging to proteins. Biochim. Biophys. Acta Proteins Proteom. 2013, 1834, 2849–2858. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Zeng, T.; Li, Y.; Liu, J.; Chen, Q.; Zhou, J.; Ye, Y.; Tang, B. Preparation of Graphene Oxide Decorated Fe3O4@SiO2 Nanocomposites with Superior Adsorption Capacity and SERS Detection for Organic Dyes. J. Nanomater. 2015, 2015, 817924. [Google Scholar] [CrossRef] [Green Version]
- Zamora-Mora, V.; Soares, P.; Echeverria, C.; Hernández, R.; Mijangos, C. Composite Chitosan/Agarose Ferrogels for Potential Applications in Magnetic Hyperthermia. Gels 2015, 1, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Huber, D.L. Synthesis, properties, and applications of iron nanoparticles. Small 2005, 1, 482–501. [Google Scholar] [CrossRef]
- Sanz, B.; Calatayud, M.P.; Cassinelli, N.; Ibarra, M.R.; Goya, G.F. Long-Term Stability and Reproducibility of Magnetic Colloids Are Key Issues for Steady Values of Specific Power Absorption over Time. Eur. J. Inorg. Chem. 2015, 2015, 4524–4531. [Google Scholar] [CrossRef] [Green Version]
- Nudelman, H.; Valverde-tercedor, C.; Kolusheva, S.; Perez, T.; Widdrat, M.; Grimberg, N.; Levi, H.; Nelkenbaum, O.; Davidov, G.; Faivre, D.; et al. Structure-function studies of the magnetite-biomineralizing magnetosome-associated protein MamC. J. Struct. Biol. 2016, 194, 1–9. [Google Scholar] [CrossRef]
- Reyes-Ortega, F.; Delgado, Á.; Schneider, E.; Checa Fernández, B.; Iglesias, G. Magnetic Nanoparticles Coated with a Thermosensitive Polymer with Hyperthermia Properties. Polymers 2017, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Mai, B.T.; Balakrishnan, P.B.; Barthel, M.J.; Piccardi, F.; Niculaes, D.; Marinaro, F.; Fernandes, S.; Curcio, A.; Kakwere, H.; Autret, G.; et al. Thermoresponsive Iron Oxide Nanocubes for an Effective Clinical Translation of Magnetic Hyperthermia and Heat-Mediated Chemotherapy. ACS Appl. Mater. Interfaces 2019, 11, 5727–5739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sola-Leyva, A.; Jabalera, Y.; Chico-Lozano, M.A.; Carrasco-Jiménez, M.P.; Iglesias, G.R.; Jimenez-Lopez, C. Reactive oxygen species (ROS) production in HepG2 cancer cell line through the application of localized alternating magnetic field. J. Mater. Chem. B 2020. [Google Scholar] [CrossRef] [PubMed]
- ISO. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Oltolina, F.; Colangelo, D.; Miletto, I.; Clemente, N.; Miola, M.; Verné, E.; Prat, M.; Follenzi, A. Tumor targeting by monoclonal antibody functionalized magnetic nanoparticles. Nanomaterials 2019, 9, 1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, M.; Ho, K.; Keating, A.; Shoichet, M.S. Doxorubicin-conjugated immuno-nanoparticles for intracellular anticancer drug delivery. Adv. Funct. Mater. 2009, 19, 1689–1696. [Google Scholar] [CrossRef]







| System | Frequency f [kHz] | SAR [W/g] | Slope dT/dt [°C/s] | ILP [nHm2kg−1] |
|---|---|---|---|---|
| M | 273 ± 5 | 68 ± 4 | 0.50 ± 0.02 | 1.6 ± 0.1 |
| 205 ± 5 | 47 ± 2 | 0.345 ± 0.009 | 1.34 ± 0.08 | |
| 163 ± 5 | 28 ± 1 | 0.202 ± 0.005 | 1.12 ± 0.06 | |
| 143 ± 5 | 24 ± 1 | 0.175 ± 0.004 | 1.09 ± 0.06 | |
| 25 TBM + 75 M | 273 ± 5 | 86 ± 4 | 0.58 ± 0.02 | 2.1 ± 0.1 |
| 205 ± 5 | 73 ± 4 | 0.50 ± 0.01 | 2.3 ± 0.1 | |
| 163 ± 5 | 54 ± 3 | 0.379 ± 0.009 | 2.1 ± 0.1 | |
| 143 ± 5 | 47 ± 3 | 0.36 ± 0.02 | 2.1 ± 0.2 | |
| 60 TBM + 40 M | 273 ± 5 | 69 ± 5 | 0.51 ± 0.02 | 1.6 ± 0.1 |
| 205 ± 5 | 56 ± 3 | 0.41 ± 0.01 | 1.8 ± 0.1 | |
| 163 ± 5 | 44 ± 3 | 0.350 ± 0.009 | 1.7 ± 0.1 | |
| 143 ± 5 | 36 ± 2 | 0.262 ± 0.007 | 1.66 ± 0.09 | |
| TBM | 273 ± 5 | 53 ± 3 | 0.37 ± 0.02 | 1.23 ± 0.07 |
| 205 ± 5 | 41 ± 2 | 0.27 ± 0.02 | 1.17 ± 0.08 | |
| 163 ± 5 | 22 ± 1 | 0.162 ± 0.009 | 0.88 ± 0.05 | |
| 143 ± 5 | 19 ± 1 | 0.134 ± 0.007 | 0.84 ± 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabalera, Y.; Oltolina, F.; Peigneux, A.; Sola-Leyva, A.; Carrasco-Jiménez, M.P.; Prat, M.; Jimenez-Lopez, C.; Iglesias, G.R. Nanoformulation Design Including MamC-Mediated Biomimetic Nanoparticles Allows the Simultaneous Application of Targeted Drug Delivery and Magnetic Hyperthermia. Polymers 2020, 12, 1832. https://doi.org/10.3390/polym12081832
Jabalera Y, Oltolina F, Peigneux A, Sola-Leyva A, Carrasco-Jiménez MP, Prat M, Jimenez-Lopez C, Iglesias GR. Nanoformulation Design Including MamC-Mediated Biomimetic Nanoparticles Allows the Simultaneous Application of Targeted Drug Delivery and Magnetic Hyperthermia. Polymers. 2020; 12(8):1832. https://doi.org/10.3390/polym12081832
Chicago/Turabian StyleJabalera, Ylenia, Francesca Oltolina, Ana Peigneux, Alberto Sola-Leyva, Maria P. Carrasco-Jiménez, Maria Prat, Concepcion Jimenez-Lopez, and Guillermo R. Iglesias. 2020. "Nanoformulation Design Including MamC-Mediated Biomimetic Nanoparticles Allows the Simultaneous Application of Targeted Drug Delivery and Magnetic Hyperthermia" Polymers 12, no. 8: 1832. https://doi.org/10.3390/polym12081832
APA StyleJabalera, Y., Oltolina, F., Peigneux, A., Sola-Leyva, A., Carrasco-Jiménez, M. P., Prat, M., Jimenez-Lopez, C., & Iglesias, G. R. (2020). Nanoformulation Design Including MamC-Mediated Biomimetic Nanoparticles Allows the Simultaneous Application of Targeted Drug Delivery and Magnetic Hyperthermia. Polymers, 12(8), 1832. https://doi.org/10.3390/polym12081832