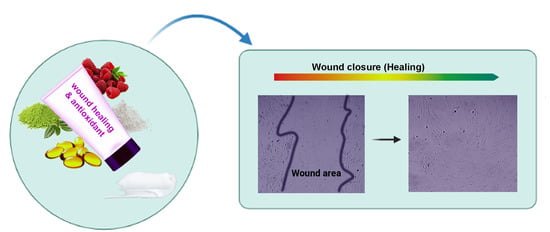
Natural Formulations Provide Antioxidant Complement to Hyaluronic Acid-Based Topical Applications Used in Wound Healing
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Formulation Preparation
2.3. Physical Assessments and pH Determination
2.4. Rheological Analysis
2.4.1. Oscillatory Rheometry
2.4.2. Continuous Shear Rheometry
2.4.3. Creep Recovery Analysis
2.5. Free Radical Scavenging Assay
2.6. Cell Culture
2.7. Cell Viability and Morphology
2.8. In Vitro Scratch Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physical Assessments and pH Determination
3.2. Rheological Properties
3.3. Antioxidant Activity
3.4. Biocompatibility and Wound Healing Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Makvandi, P.; Ghomi, M.; Padil, V.V.T.; Shalchy, F.; Ashrafizadeh, M.; Askarinejad, S.; Pourreza, N.; Zarrabi, A.; Nazarzadeh Zare, E.; Kooti, M.; et al. Biofabricated Nanostructures and Their Composites in Regenerative Medicine. ACS Appl. Nano Mater. 2020, 3, 6210–6238. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; D’Ayala, G.G. Wound healing and Antimicrobial effect of active Secondary Metabolites in Chitosan-based Wound dressings: A review. Carbohydr. Polym. 2020, 223, 115839. [Google Scholar] [CrossRef]
- Brown, M.B.; Jones, S.A. Hyaluronic acid: A unique topical vehicle for the localized delivery of drugs to the skin. J. Eur. Acad. Dermtol. Venereol. 2005, 19, 308–318. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Borzacchiello, A.; Tay, F.R.; Padil, V.V.T. Antimicrobial gum bio-based nanocomposites and their industrial and biomedical applications. Chem. Commun. 2019, 55, 14871–14885. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Tay, F.R. Recent progress in the industrial and biomedical applications of tragacanth gum. Carbohydr. Polym. 2019, 212, 450–467. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, İ.; Gökçe, E.H.; Tsapis, N.; Tanrıverdi, S.T.; Gökçe, G.; Fattal, E.; Özer, Ö. Evaluation of characteristics and in vitro antioxidant properties of RSV loaded hyaluronic acid–DPPC microparticles as a wound healing system. Colloids Surf. B Biointerfaces 2015, 126, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Wölfle, U.; Seelinger, G.; Bauer, G.; Meinke, M.C.; Lademann, J.; Schempp, C.M. Reactive molecule species and antioxidative mechanisms in normal skin and skin aging. Ski. Pharmcol. Physiol. 2014, 27, 316–332. [Google Scholar] [CrossRef]
- Xiao, Y.; Reis, L.A.; Feric, N.; Knee, E.J.; Gu, J.; Cao, S.; Laschinger, C.; Londono, C.; Antolovich, J.; McGuigan, A.P. Diabetic wound regeneration using peptide-modified hydrogels to target re-epithelialization. Proc. Natl. Acad. Sci. USA 2016, 113, E5792–E5801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, A.; Estanqueiro, M.; Oliveira, M.; Sousa Lobo, J. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, F.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B.P. Exploring the antioxidant potentiality of two food by-products into a topical cream: Stability, in vitro and in viv o evaluation. Drug Dev. Ind. Pharm. 2016, 42, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Muthukumarasamy, R.; Ilyana, A.; Fithriyaani, N.A.; Najihah, N.A.; Asyiqin, N.; Sekar, M. Formulation and evaluation of natural antioxidant cream comprising methanolic peel extract of Dimocarpus longan. Int. J. Pharm. Clin. Res. 2016, 8, 1305–1309. [Google Scholar]
- Basak, M.; Dutta, S.; Chowdhury, M. Wild raspberry: Antioxidant fruits from Eastern Himalaya. J. Food Biochem. 2018, 42, e12560. [Google Scholar] [CrossRef]
- Tripodo, G.; Trapani, A.; Torre, M.L.; Giammona, G.; Trapani, G.; Mandracchia, D. Hyaluronic acid and its derivatives in drug delivery and imaging: Recent advances and challenges. Eur. J. Pharm. Biopharm. 2015, 97, 400–416. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Thu, H.E.; Ng, S.F.; Khan, S.; Katas, H. Nanoencapsulation, an efficient and promising approach to maximize wound healing efficacy of curcumin: A review of new trends and state-of-the-art. Colloids Surf. B Biointerfaces 2017, 150, 223–241. [Google Scholar] [CrossRef]
- Vaiserman, A.; Koliada, A.; Zayachkivska, A.; Lushchak, O. Nanodelivery of natural antioxidants: An anti-aging perspective. Front. Bioeng. Biotechnol. 2019, 7, 447. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xu, P.; Yao, Z.; Fang, Q.; Feng, L.; Guo, R.; Cheng, B. Preparation of antimicrobial hyaluronic acid/quaternized chitosan hydrogels for the promotion of seawater immersion wound healing. Front. Bioeng. Biotechnol. 2019, 7, 360. [Google Scholar] [CrossRef]
- Do Amaral, R.J.F.C.; Zayed, N.M.A.; Pascu, E.I.; Cavanagh, B.; Hobbs, C.; Santarella, F.; Simpson, C.R.; Murphy, C.M.; Sridharan, R.; González-Vázquez, A. Functionalising collagen-based scaffolds with platelet-rich plasma for enhanced skin wound healing potential. Front. Bioeng. Biotechnol. 2019, 7, 371. [Google Scholar] [CrossRef] [Green Version]
- Kwak, M.S.; Ahn, H.J.; Song, K.W. Rheological investigation of body cream and body lotion in actual application conditions. Korea Aust. Rheol. J. 2015, 27, 241–251. [Google Scholar] [CrossRef]
- Konwarh, R.; Gogoi, B.; Philip, R.; Laskar, M.; Karak, N. Biomimetic preparation of polymer-supported free radical scavenging, cytocompatible and antimicrobial “green” silver nanoparticles using aqueous extract of Citrus sinensis peel. Colloids Surf. B Biointerfaces 2011, 84, 338–345. [Google Scholar] [CrossRef]
- Ferreira, A.; Vecino, X.; Ferreira, D.; Cruz, J.; Moldes, A.; Rodrigues, L. Novel cosmetic formulations containing a biosurfactant from Lactobacillus paracasei. Colloids Surf. B Biointerfaces 2017, 155, 522–529. [Google Scholar] [CrossRef] [Green Version]
- Adjimani, J.P.; Asare, P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol. Rep. 2015, 2, 721–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Kempf, M.; Kimble, R.M.; Cuttle, L. Cytotoxicity testing of burn wound dressings, ointments and creams: A method using polycarbonate cell culture inserts on a cell culture system. Burns 2011, 37, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohydr. Polym. 2019, 223, 115023–115034. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.; Elmets, C.A.; Katiyar, S.K. Green tea and skin cancer: Photoimmunology, angiogenesis and DNA repair. J. Nutr. Biochem. 2007, 18, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Madhan, B.; Krishnamoorthy, G.; Rao, J.R.; Nair, B.U. Role of green tea polyphenols in the inhibition of collagenolytic activity by collagenase. Int. J. Biol. Macromol. 2007, 41, 16–22. [Google Scholar] [CrossRef]
- An, B.J.; Kwak, J.H.; Son, J.H.; Park, J.M.; Lee, J.Y.; Park, T.S.; Kim, S.Y.; Kim, Y.S.; Jo, C.; Byun, M.W. Physiological activity of irradiated green tea polyphenol on the human skin. Am. J. Chin. Med. 2005, 33, 535–546. [Google Scholar] [CrossRef]
- Tjandra, O.; Wijayadi, L.J.; Rumawas, M.E. Green tea moisturizer improves skin hydration in elderly. Universa Med. 2018, 37, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.H.; Jung, E.Y.; Noh, D.O.; Suh, H.J. Physiological effects of formulation containing tannase-converted green tea extract on skin care: Physical stability, collagenase, elastase, and tyrosinase activities. Integr. Med. Res. 2014, 3, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Dermtol. 2012, 30, 257–262. [Google Scholar] [CrossRef]
- Ferreira, S.B.D.S.; Da Silva, J.B.; Junqueira, M.V.; Borghi-Pangoni, F.B.; Gomes, R.G.; Bruschi, M.L. The importance of the relationship between mechanical analyses and rheometry of mucoadhesive thermoresponsive polymeric materials for biomedical applications. J. Mech. Behav. Biomed. Mater. 2017, 74, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Gloria, A.; Borzacchiello, A.; Causa, F.; Ambrosio, L. Rheological characterization of hyaluronic acid derivatives as injectable materials toward nucleus pulposus regeneration. J. Biomater. Appl. 2012, 26, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Mayol, L.; Quaglia, F.; Borzacchiello, A.; Ambrosio, L.; La Rotonda, M.I. A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: Rheological, mucoadhesive and in vitro release properties. Eur. J. Pharm. Biopharm. 2008, 70, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng. C 2020, 107, 110195–110205. [Google Scholar] [CrossRef] [PubMed]
- Farahmandfar, R.; Asnaashari, M.; Salahi, M.R.; Rad, T.K. Effects of basil seed gum, Cress seed gum and Quince seed gum on the physical, textural and rheological properties of whipped cream. Int. J. Biol. Macromol. 2017, 98, 820–828. [Google Scholar] [CrossRef]
- Yang, H.; Duan, L.; Li, Q.; Tian, Z.; Li, G. Experimental and modeling investigation on the rheological behavior of collagen solution as a function of acetic acid concentration. J. Mech. Behav. Biomed. Mater. 2018, 77, 125–134. [Google Scholar] [CrossRef]
- Chhabra, R.P. Non-Newtonian fluids: An introduction. In Rheology of Complex Fluids; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–34. [Google Scholar]
- Isaac, V.L.; Moraes, J.D.; Chiari, B.G.; Guglielmi, D.A.; Cefali, L.C.; Rissi, N.C.; Corrêa, M.A. Determination of the real influence of the addition of four thickening agents in creams using rheological measurements. J. Dispers. Sci. Technol. 2013, 34, 532–538. [Google Scholar] [CrossRef]
- Gaspar, L.; Campos, P.M. Rheological behavior and the SPF of sunscreens. Int. J. Pharm. 2003, 250, 35–44. [Google Scholar] [CrossRef]
- Heberle, G.; Dos Santos, M.A.; Magri, S. Osmetic formulations containing blueberry extracts (Vaccinium myrtillus L.). TOJSAT 2012, 2, 1–6. [Google Scholar]
- Laguna, L.; Hernández, M.J.; Salvador, A.; Sanz, T. Study on resistant starch functionality in short dough biscuits by oscillatory and creep and recovery tests. Food Bioprocess Technol. 2013, 6, 1312–1320. [Google Scholar] [CrossRef]
- Zare, E.N.; Lakouraj, M.M.; Mohseni, M.; Motahari, A. Multilayered electromagnetic bionanocomposite based on alginic acid: Characterization and biological activities. Carbohydr. Polym. 2015, 130, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Lakouraj, M.M.; Moghadam, P.N.; Azimi, R. Novel polyfuran/functionalized multiwalled carbon nanotubes composites with improved conductivity: Chemical synthesis, characterization, and antioxidant activity. Polym. Compos. 2013, 34, 732–739. [Google Scholar] [CrossRef]
- Barku, V.Y. Wound healing: Contributions from plant secondary metabolite antioxidants. In Wound Healing-Current Perspectives; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Edwards, J.V.; Howley, P.; Cohen, I.K. In vitro inhibition of human neutrophil elastase by oleic acid albumin formulations from derivatized cotton wound dressings. Int. J. Pharm. 2004, 284, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.Q.; Cheng, K.J.; Qiu, J.G.; Mei, X.L.; Zhang, W.J.; Jiang, Q.W.; Qin, W.M.; Yang, Y.; Zheng, D.W.; Chen, Y. Antioxidant and Anticancer Activities of Raspberry Extracts. J. Cancer Res. Updates 2015, 4, 54–59. [Google Scholar]
- Cefali, L.C.; Franco, J.G.; Nicolini, G.F.; Ataide, J.A.; Mazzola, P.G. In vitro antioxidant activity and solar protection factor of blackberry and raspberry extracts in topical formulation. J. Cosmet. Dermtol. 2019, 18, 539–544. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 71. [Google Scholar] [CrossRef] [Green Version]
- Fernández, J.R.; Rouzard, K.; Voronkov, M.; Huber, K.L.; Webb, C.; Stock, J.B.; Stock, M.; Gordon, J.S.; Pérez, E. In vitro and clinical evaluation of SIG1273: A cosmetic functional ingredient with a broad spectrum of anti-aging and antioxidant activities. J. Cosmet. Dermtol. 2016, 15, 150–157. [Google Scholar] [CrossRef]
- Ma, W.; Wlaschek, M.; Tantcheva-Poor, I.; Schneider, L.; Naderi, L.; Razi-Wolf, Z.; Schüller, J.; Scharffetter-Kochanek, K. Chronological ageing and photoageing of the fibroblasts and the dermal connective tissue. Clin. Exp. Dermtol. 2001, 26, 592–599. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research techniques made simple: Analysis of collective cell migration using the wound healing assay. J. Investig. Dermtol. 2017, 137, e11–e16. [Google Scholar] [CrossRef] [Green Version]
- Essendoubi, M.; Gobinet, C.; Reynaud, R.; Angiboust, J.F.; Manfait, M.; Piot, O. Human skin penetration of hyaluronic acid of different molecular weights as probed by Raman spectroscopy. Ski. Res. Technol. 2016, 22, 55–62. [Google Scholar] [CrossRef]
- Jamaledin, R.; Yiu, C.K.Y.; Zare, E.N.; Niu, L.; Vecchione, R.; Chen, G.; Gu, Z.; Tay, F.R.; Makvandi, P. Advances in Antimicrobial Microneedle Patches for Combating Infections. Adv. Mater. 2020, 2002129. [Google Scholar] [CrossRef] [PubMed]









| Sample | Maximum Compliance (jmax) | Viscosity a (Pa.s) | G′ b (Pa) | G″ b (Pa) | Thixotropic Curve Area (Pa/s) |
|---|---|---|---|---|---|
| Base formulation | 0.0058 | 1.4 | 70 | 32 | 2557 |
| HBN formulation | 0.0058 | 2.5 | 1616 | 819 | 936 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makvandi, P.; Caccavale, C.; Della Sala, F.; Zeppetelli, S.; Veneziano, R.; Borzacchiello, A. Natural Formulations Provide Antioxidant Complement to Hyaluronic Acid-Based Topical Applications Used in Wound Healing. Polymers 2020, 12, 1847. https://doi.org/10.3390/polym12081847
Makvandi P, Caccavale C, Della Sala F, Zeppetelli S, Veneziano R, Borzacchiello A. Natural Formulations Provide Antioxidant Complement to Hyaluronic Acid-Based Topical Applications Used in Wound Healing. Polymers. 2020; 12(8):1847. https://doi.org/10.3390/polym12081847
Chicago/Turabian StyleMakvandi, Pooyan, Caterina Caccavale, Francesca Della Sala, Stefania Zeppetelli, Rosanna Veneziano, and Assunta Borzacchiello. 2020. "Natural Formulations Provide Antioxidant Complement to Hyaluronic Acid-Based Topical Applications Used in Wound Healing" Polymers 12, no. 8: 1847. https://doi.org/10.3390/polym12081847
APA StyleMakvandi, P., Caccavale, C., Della Sala, F., Zeppetelli, S., Veneziano, R., & Borzacchiello, A. (2020). Natural Formulations Provide Antioxidant Complement to Hyaluronic Acid-Based Topical Applications Used in Wound Healing. Polymers, 12(8), 1847. https://doi.org/10.3390/polym12081847